|
 |
- Search
| Ann Geriatr Med Res > Volume 27(4); 2023 > Article |
|
Abstract
Background
The use of ultrasonographic echo intensity (EI) to evaluate skeletal muscle quality and its effects on strength, explosive power, and physical function (PF) in older individuals remains unclear. This meta-analysis evaluated the associations among EI, muscle strength (MS), and PF in older individuals.
Methods
We conducted a systematic search of the PubMed, Embase, Web of Science, SPORT Discus, and CINAHL databases through October 2022 to identify primary studies examining the association between EI and MS/PF. Effect sizes were computed using a random-effects model and presented using forest plots. Pearson correlation coefficient (r) and I2 statistics were used to measure heterogeneity.
Results
This meta-analysis included 24 patients. EI demonstrated a negative association with maximal strength (r=-0.351; 95% confidence interval [CI], -0.411 to -0.288; p<0.001) and explosive power (r=-0.342; 95% CI, -0.517 to -0.139; p=0.001) in older individuals. Handgrip strength also showed a significant negative correlation with EI (r=-0.361; 95% CI, -0.463 to -0.249; p<0.001). However, we observed only a small and non-significant negative association between EI and gait speed (r=-0.003; 95% CI, -0.083 to -0.077; p=0.943), and a weak non-significant correlation with the chair stand test (r=0.072; 95% CI, -0.045 to 0.187; p=0.227).
Aging results in a decline in the quantity and quality of human skeletal muscle, a condition known as sarcopenia.1) Sarcopenia significantly affects the quality of life and independence of elderly adults by limiting their ability to perform daily functional activities such as standing up from a chair and walking, as well as increasing the risk of falls in this population.2,3) Ultrasound imaging is a noninvasive and safe method that can be used to assess skeletal muscle quality. Among these qualities, echo intensity (EI) is an important indicator of the proportion of noncontractile elements during aging. EI reflects the infiltration of fatty and fibrous tissue of the muscle and is quantified by examining the darkness of interest in selected areas, in which black and white indicate high and low muscle quality, respectively.4,5) The loss in muscle strength associated with skeletal muscle wastage and sarcopenia may arise from decreased muscle quality, with lower extremity strength declining more markedly than that of the upper extremities during aging, ranging from 10% to 15% loss of leg strength per decade until the age of 70 years, followed by a more rapid loss, ranging from 25% to 40% per decade.6,7)
Previous cross-sectional studies have reported significant associations between muscle quality measured using EI in the lower extremities of older adults. For instance, EI transverse images of older subjects were correlated with knee extension isometric strength (r=-0.40),8) isometric strength (r=-0.62),9) and rate of force/torque development (r=-0.39).10) In addition, quadriceps EI was negatively correlated with handgrip strength in older adults (r=-0.386).11) Interestingly, the connection of EI with muscle strength is independent of endurance and muscle size.8,9) Meanwhile, evidence has shown an inverse relationship between adiposity-to-muscle ratio assessed by ultrasound EI and functional performance in older adults, with lower EI values associated with better performance. EI is also the strongest predictor of the 30-second sit-to-stand test (30SS) (r=-0.56).12) Furthermore, EI is associated with gait-related performance, considering the role of the lower extremity muscles in locomotion. The EI of the vastus lateralis was weakly correlated with usual gait speed (USG; r=-0.05) and maximal gait speed (MGS; r=-0.11),13) while a moderate correlation was reported between the EI of the rectus femoris and USG (r=-0.46).14)
Although the available data indicate that the infiltration of noncontractile elements may affect muscle strength and functional performance in older individuals, no meta-analysis has explored the correlation between lateral EI images and muscle strength or physical function in this population. Therefore, this systematic meta-analysis investigated the associations among EI (representing muscle quality), muscle strength, and physical function in older individuals.
We conducted this review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15) The PROSPERO International Prospective Register of Systematic Reviews (CRD42023387441) registered the review protocol, to which we adhered without any deviations.
We employed a systematic search strategy using Boolean operators in the PubMed, Embase, Web of Science, SPORTDiscus, and CINAHL databases through October 2022. We modified the keywords and Boolean operators according to each database's search strategy and restricted the search to studies involving humans, written in English, and reported in peer-reviewed journals. The search strategy is presented in Supplementary Table S1.
Studies meeting the following criteria were included: (1) healthy community residents aged ≥60 years without major neurological and musculoskeletal disorders; (2) muscle mass testing using EI and reporting at least one direct assessment of muscle strength or physical function performance; (3) observational studies, including cross-sectional studies, cohort studies, and a few case-control studies; (4) published studies in English.
Articles were excluded if (1) the participants were currently on medication or had an injury that limited their physical activity and independence in daily living; (2) the study was conducted in an animal model; (3) the participants received interventions other than usual care or placebo, or randomized controlled trials; (4) the results were partially unable to extract the correlation coefficient; (5) reviews, abstracts, case reports, or duplicate published articles; and (6) non-English articles.
Two independent researchers screened the titles and abstracts of all studies based on the inclusion and exclusion criteria and then reviewed the full text of the remaining studies. Disagreements were resolved through discussion.
The data extraction process involved coding the author information, publication year, and population characteristics (sample size, sex, and mean age). The correlation coefficient (r) or standardized beta coefficient between EI and two continuous muscle strength or physical function variables was extracted. The test modality/results and the results of the muscle strength assessments and physical function tests were also coded. Muscle strength was categorized into lower extremity maximum strength (i.e., maximal voluntary force/torque of the force-/torque-time curve [MVC]), explosive force (i.e., rate of force/torque development [RFD/RTD]), and handgrip strength (assessed with a handheld dynamometer [HGS]), while physical function was divided into gait speed and mobility. Gait speed (e.g., UGS and maximum gait speed [MGS]), chair stand test (e.g., 30SS), five repetitions of the sit-to-stand test (5STS), and Timed Up-and-Go (TUG) test were used to classify physical function. If no correlation was reported, the authors were contacted for the missing information. If the author did not respond, the study was excluded from the analysis.
We assessed the risk of bias in the included studies using the Joanna Briggs Institute Analytic Cross-sectional Study Quality Checklist (Supplementary Table S2). We evaluated the methodological quality of the selected studies according to eight items that assessed the inclusion criteria, study participants and settings, criteria for condition measurement, validity and reliability of exposure and outcome measures, confounding factors and resolution strategies, and statistical analysis. Two authors evaluated each item, which was rated as “yes,” “no,” “unclear,” or “not applicable.”
We conducted the meta-analysis using Comprehensive Meta-Analysis (CMA), version 3.3.070, to analyze the Pearson product-moment correlation coefficients (r) obtained from the included studies. The r-values were converted into normally distributed variables (z-transformed Rz-values) using Fisher z-transformation according to the following formula16):
where ln is the natural logarithm.
We converted the beta coefficient (β) to an r value using the following formula17):
We calculated the weights of the studies based on standard errors (SE) using the following formula:
where N is the sample size.
We selected a random-effects model for the meta-analysis.
Correlations (positive or negative) were classified as small (r<0.3), medium (0.3≤r≤0.5), or large (r>0.5).18) We generated forest plots to display studies with 95% confidence intervals (CI) and the combined coefficients. The Rz values were reverse-converted to r values to classify and interpret relevant sizes. We evaluated the heterogeneity of the results between studies using the I2 index, where I2≤25% indicated low heterogeneity, I2>25% and I2<75% indicated moderate heterogeneity, and I2≥75% indicated high heterogeneity.19) Finally, we used funnel plots to investigate the possibility of publication bias.
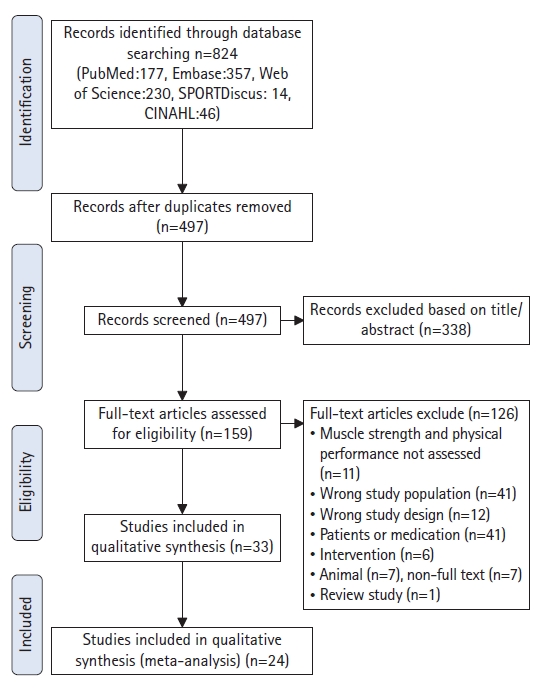
A total of 824 articles were retrieved from the initial database search through October 2022. After removing duplicates (n=327) and 338 articles based on the title or abstract, 159 articles remained and were assessed for eligibility. Finally, 24 articles were included in the meta-analysis (Fig. 1). A total of 2,501 people were included in this review, and the mean age was 71.3±5.5 years. The sample sizes ranged from 12 to 1,239. Supplementary Table S3 details the baseline characteristics of the included studies.
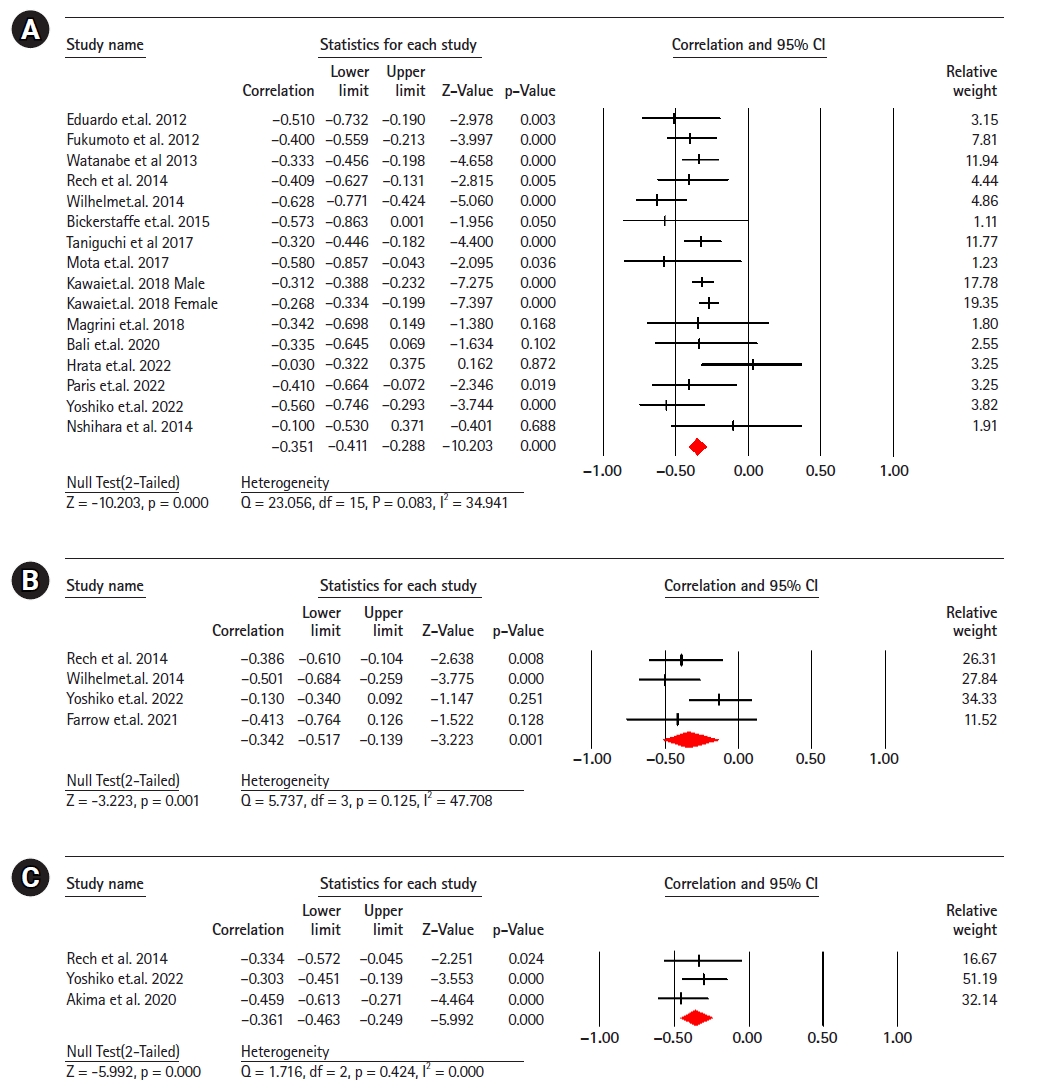
Sixteen studies (2,009 participants) analyzed the association between EI and maximal strength in healthy older adults.8,9,11,13,20-30) The results revealed a significant moderate correlation negative between EI and maximum strength (r=-0.35; 95% CI, -0.41 to -0.28; p<0.001; I2=34.94). Four studies (190 participants) analyzing the association between EI and explosive power in healthy older adults,11,22,28,31) showed a significant moderate negative correlation between EI and explosive power (r=-0.34; 95% CI, -0.51 to -0.13; p=0.001; I2=47.70). Three studies (261 participants) showed a moderate negative correlation between EI and handgrip strength11,32,33) (r=-0.36; 95% CI, -0.46 to -0.24; p<0.001; I2=0.000) (Fig. 2).
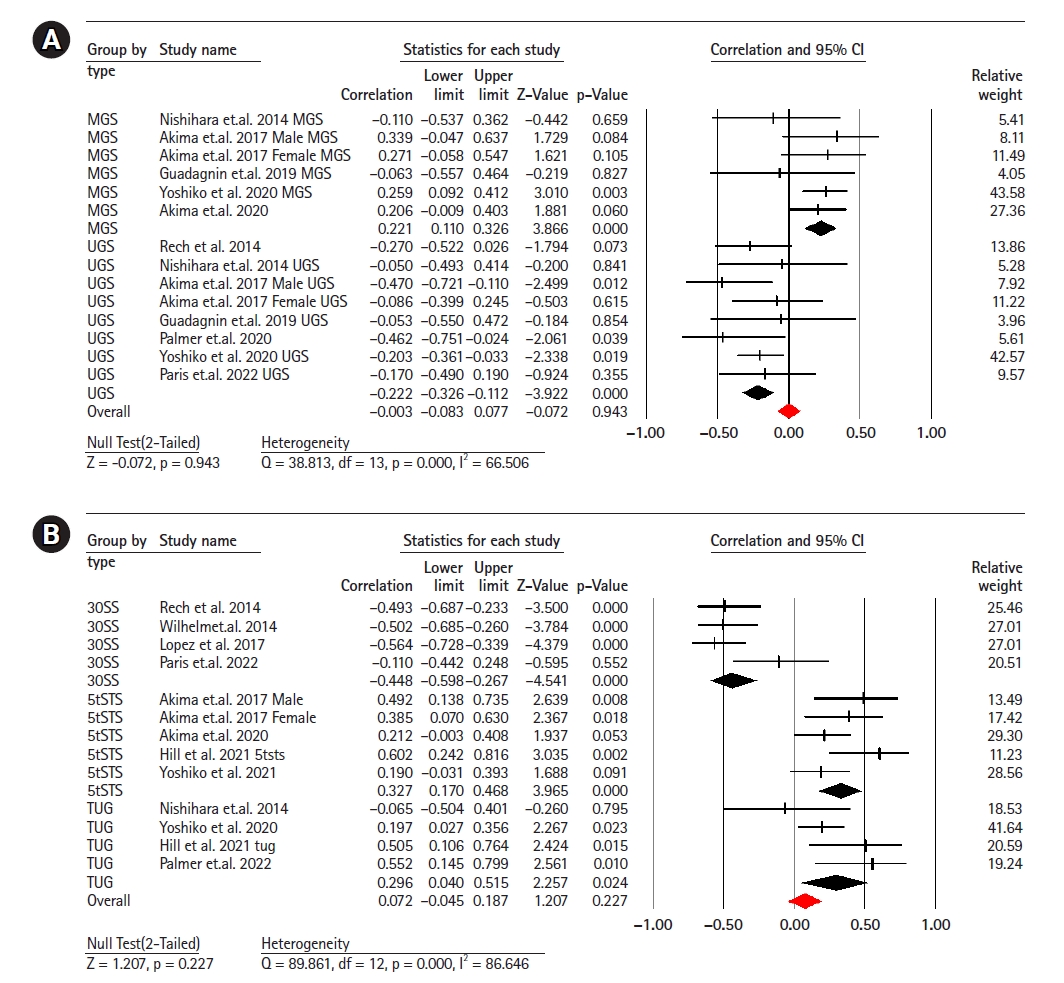
Fourteen studies (involving 641 participants) investigated the association between EI and gait speed.11,13,14,21,22,33-35) The combined effect size for EI and gait speed was r=0.00 (95% CI, -0.08 to -0.07; p=0.94; I2=66.50), indicating no linear correlation between the two with moderate heterogeneity. Subgroup analysis showed a weak negative correlation between UGS and EI (r=-0.22; 95% CI, -0.32 to -0.11; p<0.001; I2=0.00), while there was a weak positive correlation between MGS and EI (r=0.22; 95% CI, 0.11 to 0.32; p<0.001; I2=0.00) (Fig. 3).
Thirteen studies (620 participants) investigated the association between EI and the chair stand test.11-14,21,29,32,35-37) The combined effect size of EI and 30SS was r=0.07 (95% CI, -0.04 to 0.18; p=0.22; I2=86.64), with a weak statistical correlation and considerable heterogeneity. Subgroup analyses showed a moderate negative correlation between EI and the 30SS test (r=-0.44; 95% CI, -0.59 to -0.26; p<0.001; I2=45.41) and between EI and the 5STS test (r=0.32; 95% CI, 0.17 to 0.46; p<0.001; I2=35.42), respectively. We observed a weak positive correlation between EI and the TUG test (r=0.29; 95% CI, 0.04 to 0.21; p=0.02; I2=25.42) (Fig. 3).
The relative symmetry displayed in Fig. 3A indicates no apparent publication bias. Visual inspection of the funnel plot in Fig. 3B suggested insufficient evidence of publication bias, with an intercept result of 1.03 (SE=2.10; 95% CI, -3.51 to 5.58; t=0.48; df=13; p=0.63), indicating no strong evidence of publication bias (Supplementary Fig. S1).
This meta-analysis examined the correlation between EI in the thigh muscles, muscle strength, and physical functional performance in healthy older individuals.
Our results revealed a significant moderate inverse correlation between EI and maximal strength in the lower extremities, explosive power, and handgrip strength in this population. However, the meta-analysis showed contradictory evidence for the association between EI and physical functional performance, which appeared to be task-specific. In summary, the strength of the association between thigh EI and physical function may depend on the specific type of physical function test used.
The results of our meta-analysis suggested a moderately negative correlation between EI and muscle strength in older adults. Muscle quality, which is related to the amounts of muscle fiber and fat tissue, is an independent determinant of muscle strength. Gray-scale analysis of EI is a valuable tool for assessing muscle strength.5) Furthermore, the relationships between EI and different types of strength exhibit unique features. EI and maximum strength are negatively correlated (r=-0.3), with the fat and connective tissue in muscles playing a significant role in isometric and isokinetic strength in older adults. This finding is consistent with those of previous studies showing a correlation between the EI of the rectus femoris muscle and isometric/isokinetic peak torque, with r values ranging from -0.40 to -0.67.8,30) However, contrary to our study, these previous studies observed no relationship between thigh EI and maximal isometric strength.38) One explanation for this discrepancy could be the use of different measurement techniques to assess EI. In EI determined from transverse images, the ultrasound probe positioning significantly affects the results.5) Therefore, discrepant findings in the literature may be due to differences in probe orientation. The current evidence suggests that the accumulation of non-contractile components in thigh muscles significantly affects maximal knee extension and flexion strength in older adults.28)
A previous study showed that older adults experience a more significant decline in explosive speed than in maximum muscle velocity.39) Our results demonstrated a moderate correlation (r=-0.3) between EI and knee explosive power, which can be attributed to the increased intramuscular fat infiltration associated with aging. This change leads to a decrease in single-fiber contraction velocity and power output, alters mechanical muscle properties, increases muscle stiffness, and alters fiber shortening and bulging.8,40) Additional neuromuscular variables contribute to the age-related decrease in explosive speed; specifically, the fast performance of older adults may be influenced by motor unit firing rate.41) In addition, the decline in muscle strength associated with aging may be owing to factors beyond muscle mass, such as decreased proportion of fast type II fibers and reduced muscle excitatory neural activation.42-44) Moreover, coactivation, which refers to opposing muscle mechanical action, is higher in older adults, resulting in reduced force production.45)
The assessment of grip strength using a HGS is a practical approach for evaluating muscle strength in clinical contexts.46) In addition to its ease of application, grip strength can serve as a crucial indicator of physical functionality and is associated with mortality rates in certain pathological conditions. Our meta-analysis identified a moderate correlation (r=-0.3) between EI strength and handgrip strength, consistent with the outcomes of various previous studies.11,32,33)
Although we did not observe a significant association between EI and gait speed, subgroup analyses revealed a weak association between maximal and usual gait speeds. Previous research on older adults showed no significant correlation between the muscle EI of the quadriceps femoris and the 6-minute walk, which was attributed to an increase in subcutaneous fat thickness. However, the relationship became statistically significant after adjusting for subcutaneous fat.34) These findings raise concerns about whether it is necessary to adjust for subcutaneous fat thickness in EI measurements for older adults.
We examined the relationship between chair-stand performance and EI. We observed a weak correlation between chair-stand performance and EI, with substantial heterogeneity. Previous studies comparing various types of chair tests have reported that a 30-second chair stand is the optimal parameter for predicting EI in older adults.36) Subgroup analysis revealed a moderate correlation (r=0.4) between 30-second chair stand performance and EI, supporting the previous finding that an increased proportion of non-contractile elements may lead to functional status deterioration with aging.4) The high heterogeneity in the meta-analysis may have resulted from using different cutoff points. Age is a primary factor affecting chair-stand performance and EI and may confound the assessment of this association. Moreover, ankle plantar flexors exhibit a similarly strong association (r=0.45–0.59) with chair function tests in older adults,37) implying that muscle type may account for the lack of significant association between chair-stand tests and EI in our study.
In addition, EI obtained using ultrasound may be influenced by methodological factors such as subcutaneous fat correction, biological factors such as sex and race, and environmental factors such as daily physical activity and exercise,5,47,48) which cannot be completely controlled in clinical settings. The inconsistency between the results of our meta-analysis and those of previous studies emphasizes the need to carefully consider confounding factors when examining the relationship between EI and physical function.
This study had several limitations. First, due to the lack of a standardized EI measurement method, we used raw EI data. Subcutaneous fat thickness may attenuate ultrasound findings and affect the reliability of muscle EI results. Second, insufficient data were available to perform a meta-analysis of muscles outside the thigh; therefore, the analysis does not represent the strength and overall function of the lower limb muscles. In addition, not all the studies controlled for confounding variables. Although this report examined the results in older adults, the included studies did not separately investigate participants by sex; therefore, potential differences between the sexes are unknown. Finally, the current meta-analysis was based on cross-sectional data; thus, the association does not imply causality. Therefore, the relationship between muscle structural characteristics, muscle strength, and physical function variables could not be determined.
Overall, our meta-analysis results support EI as an effective indicator for evaluating muscle strength and physical functional performance; however, the influence of factors such as different muscle types, age, and sex must be considered. Future research should explore the impact of these factors on this relationship to better understand the application of echogenicity in evaluating muscle strength and functional performance.
Our findings suggest that increased EI in the thigh muscles is associated with decreased strength and power in older individuals. However, we did not observe a significant association between EI and gait speed or mobility. Further well-designed studies with larger sample sizes and longer-term follow-ups are required to validate the practical implications of these results in predicting frailty and assessing risks in this population.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4235/agmr.23.0101.
Table S3.
Characteristics of included studies and measured activities of muscle quality, muscle strength and physical function
Fig. S1.
Publication bias funnel plot for research studies reporting the relationship between echo intensity and gait speed (A), and chair stand test (B).
REFERENCES
3. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 2006;119:526.



4. Miron Mombiela R, Facal de Castro F, Moreno P, Borras C. Ultrasonic echo intensity as a new noninvasive in vivo biomarker of frailty. J Am Geriatr Soc 2017;65:2685–90.



5. Stock MS, Thompson BJ. Echo intensity as an indicator of skeletal muscle quality: applications, methodology, and future directions. Eur J Appl Physiol 2021;121:369–80.


6. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–64.


7. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 2001;56:B209–17.



8. Fukumoto Y, Ikezoe T, Yamada Y, Tsukagoshi R, Nakamura M, Mori N, et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol 2012;112:1519–25.


9. Mota JA, Stock MS. Rectus femoris echo intensity correlates with muscle strength, but not endurance, in younger and older men. Ultrasound Med Biol 2017;43:1651–7.


10. Gerstner GR, Thompson BJ, Rosenberg JG, Sobolewski EJ, Scharville MJ, Ryan ED. Neural and muscular contributions to the age-related reductions in rapid strength. Med Sci Sports Exerc 2017;49:1331–9.



11. Rech A, Radaelli R, Goltz FR, Rosa LH, Schneider CD, Pinto RS. Echo intensity independently predicts functionality in sedentary older men. Muscle Nerve 2017;55:9–15.



12. Lopez P, Wilhelm EN, Rech A, Minozzo F, Radaelli R, Pinto RS. Echo intensity independently predicts functionality in sedentary older men. Muscle Nerve 2017;55(1):9–15.



13. Nishihara K, Kawai H, Hayashi H, Naruse H, Kimura A, Gomi T, et al. Frequency analysis of ultrasonic echo intensities of the skeletal muscle in elderly and young individualss. Clin Interv Aging 2014;9:1471–8.



14. Palmer TB, Farrow AC. Correcting for subcutaneous fat: does it improve the correlation between vastus lateralis echo intensity and physical performance in older women? Clin Physiol Funct Imaging 2022;42:372–9.



15. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9.




16. Fisher RA. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika 1915;10:507–21.

17. Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. J Appl Psychol 2005;90:175–81.


18. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 2012;141:2–18.


19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.



20. Hirata K, Ito M, Nomura Y, Kawashima C, Tsuchiya Y, Ooba K, et al. Muscle quality indices separately associate with joint-level power-related measures of the knee extensors in older males. Eur J Appl Physiol 2022;122:2271–81.




21. Paris MT, Bell KE, Avrutin E, Mourtzakis M. Association of strength, power, and function with muscle thickness, echo intensity, and lean tissue in older males. Appl Physiol Nutr Metab 2022;47:521–8.


22. Yoshiko A, Beppu M, Chosa N, Watanabe K. Unique characteristics of quadriceps muscle morphology and function in older tennis players. J Aging Phys Act 2021;30:697–704.


23. Bali AU, Harmon KK, Burton AM, Phan DC, Mercer NE, Lawless NW, et al. Muscle strength, not age, explains unique variance in echo intensity. Exp Gerontol 2020;139:111047.


24. Kawai H, Kera T, Hirayama R, Hirano H, Fujiwara Y, Ihara K, et al. Morphological and qualitative characteristics of the quadriceps muscle of community-dwelling older adults based on ultrasound imaging: classification using latent class analysis. Aging Clin Exp Res 2018;30:283–91.



25. Magrini MA, Colquhoun RJ, Barrera-Curiel A, Thiele RM, DeFreitas JM, Smith DB, et al. Muscle size, strength, power, and echo intensity, but not specific tension, are affected by age in physically active adults. Isokinet Exerc Sci 2018;26:95–103.

26. Taniguchi M, Yamada Y, Fukumoto Y, Sawano S, Minami S, Ikezoe T, et al. Increase in echo intensity and extracellular-to-intracellular water ratio is independently associated with muscle weakness in elderly women. Eur J Appl Physiol 2017;117:2001–7.



27. Bickerstaffe A, Beelen A, Zwarts MJ, Nollet F, van Dijk JP. Quantitative muscle ultrasound and quadriceps strength in patients with post-polio syndrome. Muscle Nerve 2015;51:24–9.



28. Wilhelm EN, Rech A, Minozzo F, Radaelli R, Botton CE, Pinto RS. Relationship between quadriceps femoris echo intensity, muscle power, and functional capacity of older men. Age (Dordr) 2014;36:9625.




29. Watanabe Y, Yamada Y, Fukumoto Y, Ishihara T, Yokoyama K, Yoshida T, et al. Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin Interv Aging 2013;8:993–8.



30. Cadore EL, Izquierdo M, Conceicao M, Radaelli R, Pinto RS, Baroni BM, et al. Echo intensity is associated with skeletal muscle power and cardiovascular performance in elderly men. Exp Gerontol 2012;47:473–8.


31. Farrow AC, Palmer TB. Age-related differences in hip flexion maximal and rapid strength and rectus femoris muscle size and composition. J Appl Biomech 2021;37:311–9.


32. Akima H, Yoshiko A, Radaelli R, Ogawa M, Shimizu K, Tomita A, et al. Comparison of muscle quality and functional capacity between Japanese and Brazilian older individuals. PLoS One 2020;15:e0243589.



33. Akima H, Yoshiko A, Ogawa M, Maeda H, Tomita A, Ando R, et al. Quadriceps echo intensity can be an index of muscle size regardless of age in 65 or more years old. Exp Gerontol 2020;138:111015.


34. Guadagnin EC, Priario LA, Carpes FP, Vaz MA. Correlation between lower limb isometric strength and muscle structure with normal and challenged gait performance in older adults. Gait Posture 2019;73:101–7.


35. Akima H, Yoshiko A, Tomita A, Ando R, Saito A, Ogawa M, et al. Relationship between quadriceps echo intensity and functional and morphological characteristics in older men and women. Arch Gerontol Geriatr 2017;70:105–11.


36. Yoshiko A, Ogawa M, Shimizu K, Radaelli R, Neske R, Maeda H, et al. Chair sit-to-stand performance is associated with diagnostic features of sarcopenia in older men and women. Arch Gerontol Geriatr 2021;96:104463.


37. Hill MW, Roberts M, Price MJ, Kay AD. Association between knee extensor and ankle plantarflexor muscle thickness and echo intensity with postural sway, mobility and physical function in older adults. Exp Gerontol 2021;150:111385.


38. Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr) 2013;35:2377–88.




39. Thompson BJ, Conchola EC, Palmer TB, Stock MS. Effects of aging on maximal and rapid velocity capacities of the leg extensors. Exp Gerontol 2014;58:128–31.


40. Choi SJ, Files DC, Zhang T, Wang ZM, Messi ML, Gregory H, et al. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci 2016;71:557–64.



41. Rahemi H, Nigam N, Wakeling JM. The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. J R Soc Interface 2015;12:20150365.




42. Stevens JE, Binder-Macleod S, Snyder-Mackler L. Characterization of the human quadriceps muscle in active elders. Arch Phys Med Rehabil 2001;82:973–8.


43. Sipila S, Koskinen SO, Taaffe DR, Takala TE, Cheng S, Rantanen T, et al. Determinants of lower-body muscle power in early postmenopausal women. J Am Geriatr Soc 2004;52:939–44.


44. McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 2014;3:9.




45. Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve 2002;25:858–63.


46. Jakobsen LH, Rask IK, Kondrup J. Validation of handgrip strength and endurance as a measure of physical function and quality of life in healthy subjects and patients. Nutrition 2010;26:542–50.


- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 1,261 View
- 170 Download
-
Related articles in
Ann Geriatr Med Res