|
 |
- Search
| Ann Geriatr Med Res > Volume 25(1); 2021 > Article |
|
Abstract
Background
With the rapid demographic change in Korea, Alzheimer’s disease has become a primary concern. Recent developments in disease-modifying therapies provide hope that therapy may become available soon. The high disease prevalence and complex evaluation process will create challenges for the healthcare system already burdened by the current pandemic. This study examined the preparedness of the South Korean healthcare system to identify and treat patients when such a therapy becomes available.
Methods
We used a Markov model to simulate a stylized patient’s journey. Based on national data and expert input, we presented projections of the diagnosis and treatment wait times and respective queues of patients under treatment and no-treatment scenarios and further simulated the possible option of adopting a blood-based biomarker test.
Results
Under the current system, we estimated a peak waiting time of 14 months when a treatment became available, largely because of the limited number of dementia specialists. Adopting a blood-based biomarker test dramatically reduced the initial wait times by more than half. A disease-modifying therapy was estimated to avert 575,000 incident cases in the first 10 years after the treatment entered the market, and a blood-based test further avoided 86,000 additional cases.
Conclusion
South Korea’s healthcare infrastructure requires more preparation for the introduction of a disease-modifying therapy, with the primary capacity limitation being the low number of dementia specialists. The utilization of a blood-based test for Alzheimer’s disease biomarkers may be an effective solution.
Alzheimer’s disease is a rapidly growing concern in Korea because it is the fastest aging country among the Organisation for Economic Co-operation and Development (OECD) countries, with projections showing it possibly becoming the country with the highest number of older people in 2075.1) As cognitive decline increases with age, the prevalence of the disease is bound to grow with demographic change. In 2018, approximately 750,000 older Koreans had been diagnosed with dementia, with 75% being due to Alzheimer’s disease. This number is expected to increase to 3 million by 2050,2) and 1.7 million Koreans are estimated to currently live with mild cognitive impairment (MCI).3) In response, various therapies aiming to prevent, delay the onset, and/or slow the progression of Alzheimer’s disease are being investigated. While numerous clinical trials have failed, recent progress provides hope for the possibility of the first disease-modifying therapy to become available in 2021.4)
However, the introduction of such a treatment will create challenges for the healthcare system, especially in light of the coronavirus disease 2019 (COVID-19) pandemic, thus creating competing priorities.5) A disease-modifying therapy will likely be prescribed to patients in the early stages of the disease to slow its progression. Identifying treatment-eligible patients requires a complex evaluation process, including brief cognitive assessment, confirmatory neurocognitive evaluation, and biomarker tests. Fortunately, the Korean government has been implementing a series of national dementia strategies since 2008. In 2017, the current administration announced the National Dementia Responsibility Policy, in which the country should bear most of the burden of dementia care.6) The policy established regional dementia centers that provide free consultation and cognitive screening for dementia patients and family members.7)
This study analyzed the preparedness of the Korean healthcare system when a disease-modifying therapy for Alzheimer’s disease becomes available. We simulated the identification and treatment processes of people with early-stage Alzheimer’s disease under a treatment compared to a no-treatment status and presented projections of the potential wait times in Korea. We further simulated the effect of adopting a blood-based biomarker test for Alzheimer’s disease pathology.
Our simulation model used a patient journey on the path to a disease-modifying therapy as our basis. Individuals aged 50 years and over go through a cognitive assessment (screening phase), where those detected with MCI are referred to a dementia specialist for further analysis (diagnostic phase), and if confirmed with a positive biomarker test, they are referred to treatment (treatment phase). For individuals who are untreated, the disease continues to progress. We applied this path to a Markov model and transition probabilities derived from the literature,8,9) where individuals moved through the disease states: normal cognition to MCI to Alzheimer’s disease. To simulate the healthcare system, our model included three capacity constraints: dementia specialists, biomarker testing facilities, and treatment delivery facilities. The details of the model can be found in previous studies, in which we analyzed the preparedness of the healthcare systems in the United States, Australia, Canada, Japan, and six European countries (Germany, France, Italy, Spain, Sweden, and the UK).10-14)
Our population projections and mortality rates were obtained from the Korean Census,15) and similar to our previous research, estimations of those for people with MCI and Alzheimer’s disease were derived from information from the literature.16-20) Based on expert input from Korean subject-matter experts, we assumed that 80% of neurologists and 35% of psychiatrists would evaluate patients with MCI and Alzheimer’s disease. Regarding biomarker tests in Korea, we assumed that the majority (95%) of tests would be performed using positron emission tomography (PET) for amyloid deposits in the brain and the remaining via cerebrospinal fluid (CSF) tests owing to the strong reluctance of Koreans to undergo lumbar puncture. Our model did not apply any constraint on CSF tests, while the capacity was constrained for PET given the limited excess capacity and a slight decrease in the number of devices. The numbers of specialists and PET scanners were derived from Korea’s Health Insurance Review & Assessment Service and OECD Health Statistics.21,22) We projected future capacities using the historical trends of the physician workforce and PET scanners. For the treatment phase, we assumed that a disease-modifying therapy would be intravenously delivered and reduce the risk of progression from MCI to dementia by 30%. Because of the lack of infusion data in Korea, we used a healthcare system capacity index for infusion projections using OECD data,21) consistent with our previous analyses.
Our model started with individuals aged 50 years and older because most later-stage clinical trials included individuals with ages as low as 50 years (e.g., the Phase 3 trial of BAN2401, NCT03887455). We assumed that a disease-modifying therapy for patients with MCI due to Alzheimer’s disease would become available in 2022 and that screening would start 1 year earlier in 2021. The treatment was assumed to be delivered via intravenous infusion every 4 weeks for 18 months following the protocol for aducanumab. Our Korean model applied the same assumptions for treatment effectiveness, patient uptake, excess capacity, and epidemiological parameters as in our previous studies but modified them for the Korean context. We consulted several experts familiar with the Korean subject matter to adapt these assumptions. Details on the model assumptions are described in our previous studies.10-14)
Several blood-based test kits for Alzheimer’s disease have emerged in Korea,23) and in 2020, a test in which a multimer detection system (MDS) detects plasma Aβ oligomers,24) with a discriminative accuracy comparable to an amyloid PET,25) the “MDS-OAβ test,” received approval from the Korean Food & Drug Administration (FDA). The introduction of a blood-based biomarker test for Alzheimer’s disease would allow the identification of patients with MCI due to other causes earlier in the process. A study using US data showed that such a test could lead to an approximate reduction in specialist visits by 60% and the use of PET by 40%.26) Based on this study, our model reflected the possible introduction of such a blood-based test to analyze its potential effect on clearing wait times and respective queues.
Based on our analysis, 1.8 million Koreans are estimated to have MCI in 2021. Among them, 0.9 million are assumed to seek further evaluation by a dementia specialist, of whom 0.8 million would undergo biomarker tests, with half of the patients expected to test positive for amyloid pathology, resulting in 0.3 million patients eligible for treatment.
The projected number of patients in queues for a disease-modifying therapy and respective wait times are presented in Fig. 1. The initial peak wait time was estimated to be 14 months, with the main capacity limitation being specialist visits. Initially, over 500,000 Korean patients were estimated to wait for their specialist appointment. Infusion delivery wait times were minimal and would persist until 2028. The backlog of cases was projected to clear in 2029 when patients could access treatment without waiting. We did not project waitlists for biomarker tests because of the relatively high PET scanner capacity in Korea.
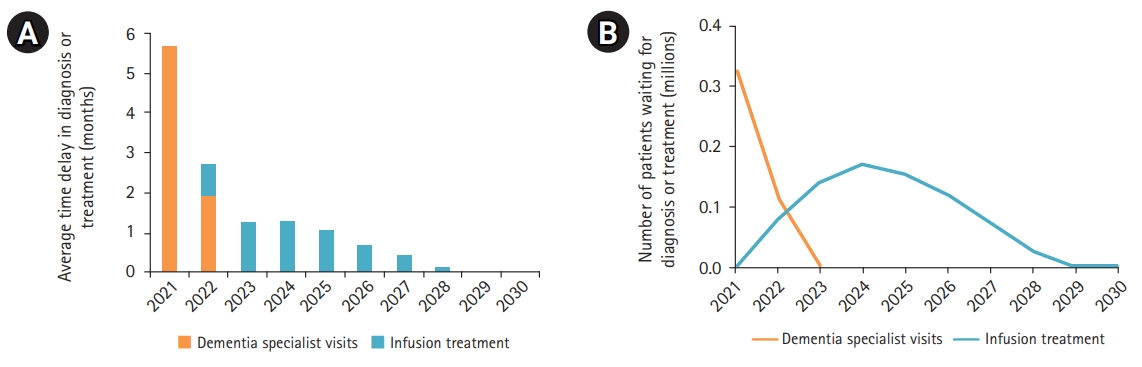
Fig. 2 shows that a blood-based biomarker test would reduce the initial wait times by more than half, to 5.7 months, with queues for specialist visits clearing in 2023. While the increased throughput in the screening phase would result in more patients waiting for infusions, the net effect was lower wait times and 620,000 fewer patients waiting in queues that would persist until 2028. Cumulatively, 769,000 patients would wait for infusion treatment from 2021 to 2029, with a peak of 171,000 people in queue in 2024.
Fig. 3 further compares the projected incident cases of Alzheimer’s disease up to 2050 under three scenarios: without treatment (gray dashed line) and with treatment assuming that a blood test is not (blue solid line) and is (orange line with markers) available. Compared to the cumulative number of new cases in the absence of a treatment, the disease-modifying therapy helped avoid approximately 575,000 additional cases in the first 10 years after the entry of a treatment in the market, assuming our projected wait times in the absence of a blood test. Assuming the availability of a blood-based test, an additional 86,000 incident cases could be averted owing to faster access to treatment. If we increased the projection period to 2050, the disease-modifying therapy could help avert 2.6 million new cases compared to no treatment and the blood-based test would avert further 92,000 additional cases.
A disease-modifying therapy for Alzheimer’s disease may soon become available for the first time. Such therapy may reduce the number of patients with dementia due to Alzheimer’s disease by delaying or preventing disease progression. However, this preventive paradigm implies that the population effect of a therapy will depend on a country’s ability to identify patients eligible for treatment and to administer it in a timely manner. Waiting times persisted for a decade, with the annual average wait times peaking at 14 months. The projected peak wait time was similar to that in Japan (15 months) and the UK (14 months) and shorter than that in the United States (19 months) and Canada (28 months).10-14)
The limited capacity of dementia specialists is the biggest obstacle in evaluating patients with MCI in Korea. Geriatric medicine is yet to be established as an official specialty in Korea, with a negligible number of physicians specializing in dementia. For instance, approximately 6,000 members participated in the Korean Geriatric Society in 2010, mostly from internal and family medicine.27) Expanding the number of specialists is also the most challenging constraint to address because of the long training times and overall limited number of physicians.
Fortunately, blood-based tests for Alzheimer’s disease pathology are the functional equivalent of increasing specialist capacity by 60% because they improve the efficiency of the initial triage process. The currently available cognitive tests have limited specificity for discerning MCI due to Alzheimer’s disease from MCI due to other etiologies.28) Blood-based tests in combination with a brief cognitive test, however, may help prioritize patients with likely MCI due to Alzheimer’s disease at the primary care level for specialist referral and reduce the need for confirmatory biomarker tests. The recently approved test kit in Korea has been demonstrated to be a reliable method for evaluation of individuals with Alzheimer’s disease.23-25)
Eliminating the constraint for infusion delivery is also needed. This could be achieved by increasing the number of hospital beds for infusion delivery. In the long term, home infusions may become available, especially in rural areas, which can further reduce related wait times. At-home services organized by regional dementia centers may include home infusions in the future.
Our analysis had several limitations; thus, our estimates represented the magnitude of the problem rather than an exact projection of wait times and disease progression. We relied on several assumptions to identify potential capacity constraints if a disease-modifying therapy becomes available. These assumptions were based on the blood-based biomarker test reducing capacity constraints from an example of the United States case; however, the effect may differ in the Korean system using a Korean test kit. The study using US data was based on a test detecting beta-amyloid 42 and 40 (Aβ42 and Aβ40) with a sensitivity and specificity of 0.89 and 0.69, respectively.29) The Korean test kit detects plasma Aβ oligomers and also incubates plasma samples with synthesized Aβ42 before the assay. The tests show promise, with a sensitivity of 78.3% and a specificity of 86.5%.30) In our study, we assumed that 80% of neurologists and 35% of psychiatrists would be able to diagnose patients. This does not reflect the smaller proportion of physicians currently specializing in dementia care but rather the proportion of experts we considered would be capable of evaluating patients with cognitive decline. Using the actual proportion of dementia specialists would lead to higher wait times. We did not consider capacity challenges related to cognitive screening, magnetic resonance imaging, radiologists and nuclear medicine specialists, and treatment monitoring owing to the complexity of the model. Our focus on dementia specialists, biomarker testing for diagnosis, and infusion delivery reflected the fact that these are likely to be the most crucial and pressing barriers to overcome with the introduction of a disease-modifying therapy.
In conclusion, there is cautious optimism that a disease-modifying therapy for Alzheimer’s disease will soon be available. Similar to many other countries, Korea does not yet have sufficient infrastructure to deliver such a therapy to a large pool of prevalent patients, leading to initial wait times for access to treatment. Utilizing a blood-based biomarker test for the early diagnosis of Alzheimer’s disease may help accelerate the evaluation of treatment-eligible patients, thereby preventing disease progression to manifest dementia in a greater number of patients.
ACKNOWLEDGEMENTS
For this analysis, we consulted subject-matter experts to inform our modeling assumptions. We thank these experts for sharing their insights into clinical practices and policies in Korea, including Hee-Jin Kim (Hanyang University), Hee-Jin Kim (Sungkyunkwan University), Seung-Ho Ryu (Konkuk University), and Seok Woo Moon (Konkuk University). Their willingness to be acknowledged does not imply their agreement with the report’s assumptions and conclusions.
ACKNOWLEDGMENTS
CONFLICT OF INTEREST
Soeren Mattke serves on the board of directors of Senscio Systems Inc. and the scientific advisory boards of AiCure Technologies, Boston Millennia Partners, and Zano Zano Healthcare Services. He has received consulting fees from AARP, Biotronik, Bristol-Myers Squibb, Eisai, and Defined Health. Except for that, no potential conflict of interest relevant to this article was reported.
REFERENCES
1. Organisation for Economic Cooperation and Development. Pensions at a Glance 2017: OECD and G20 Indicators. Paris, France: OECD Publishing; 2017.
2. Cho H, Kim J. National Institute of Dementia Annual Report 2019 [Internet]. Seoul, Korea: National Institute of Dementia; 2020 [cited 2021 Jan 30]. Available from: https://www.nid.or.kr/notification/activity_view.aspx?board_seq=1983&page&searchfield&searchword.
3. Lee J, Kang M, Nam H, Kim Y, Lee O, Kim K. Korean Dementia Observatory 2019 [Internet]. Seoul, Korea: National Institute of Dementia; 2020 [cited 2021 Jan 30]. Available from: https://www.nid.or.kr/info/dataroom_view.aspx?bid=209.
4. Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2020. Alzheimers Dement (N Y) 2020;6:e12050.



5. Lekamwasam R, Lekamwasam S. Effects of COVID-19 pandemic on health and wellbeing of older people: a comprehensive review. Ann Geriatr Med Res 2020;24:166–72.



6. Lee DW, Seong SJ. Korean national dementia plans: from 1st to 3rd. J Korean Med Assoc 2018;61:298–303.

7. Lee SB. The Community Dementia Reassurance Center (Chime Ansim Center) in South Korea. Ann Geriatr Med Res 2019;23:43–4.



8. Yesavage JA, O'Hara R, Kraemer H, Noda A, Taylor JL, Ferris S, et al. Modeling the prevalence and incidence of Alzheimer's disease and mild cognitive impairment. J Psychiatr Res 2002;36:281–6.


9. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia: meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 2009;119:252–65.


10. Liu J, Hlavka J, Hillestad RJ, Mattke S. Assessing the preparedness of the US health care system infrastructure for an Alzheimer's treatment. Santa Monica, CA: RAND Corporation; 2017.
11. Hlavka JP, Mattke S, Liu JL. Assessing the preparedness of the health care system infrastructure in six European countries for an Alzheimer's treatment. Santa Monica, CA: RAND Corporation; 2018.
12. Liu JL, Hlavka JP, Coulter DT, Baxi SM, Mattke S, Gidengil CA. Assessing the preparedness of the Canadian health care system infrastructure for an Alzheimer's treatment. Santa Monica, CA: RAND Corporation; 2019.
13. Mattke S, Hlavka JP, Yoong J, Wang M, Goto R. Assessing the preparedness of the Japanese health care system infrastructure for an Alzheimer’s treatment. Los Angeles, CA: Center for Economic and Social Research; 2019 [cited 2021 Jan 30]. Available from: https://cesr.usc.edu/sites/default/files/Japan_Infrastructure_Report_Update_f2.pdf.

14. Baxi SM, Girosi F, Liu JL. Assessing the preparedness of the Australian health care system infrastructure for an Alzheimer's disease-modifying therapy. Santa Monica, CA: RAND Corporation; 2019.
15. Statistics Korea. Population trend survey (mortality rate statistics) [Internet]. Daejeon, Korea: Statistics Korea; c2020 [cited 2021 Jan 30]. Available from: http://kostat.go.kr/portal/korea/kor_nw/1/2/3/index.board.
16. Petersen RC, Lopez O, Armstrong MJ, Getchius TS, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018;90:126–35.



17. Montgomery W, Ueda K, Jorgensen M, Stathis S, Cheng Y, Nakamura T. Epidemiology, associated burden, and current clinical practice for the diagnosis and management of Alzheimer's disease in Japan. Clinicoecon Outcomes Res 2017;10:13–28.


18. Vassilaki M, Cha RH, Aakre JA, Therneau TM, Geda YE, Mielke MM, et al. Mortality in mild cognitive impairment varies by subtype, sex, and lifestyle factors: the Mayo Clinic Study of Aging. J Alzheimers Dis 2015;45:1237–45.



19. Spackman DE, Kadiyala S, Neumann PJ, Veenstra DL, Sullivan SD. Measuring Alzheimer disease progression with transition probabilities: estimates from NACC-UDS. Curr Alzheimer Res 2012;9:1050–8.



20. Rossetti HC, Munro Cullum C, Hynan LS, Lacritz LH. The CERAD Neuropsychologic Battery Total Score and the progression of Alzheimer disease. Alzheimer Dis Assoc Disord 2010;24:138–42.



21. Health Insurance Review & Assessment Service. Healthcare Bigdata Hub [Internet]. Wonju, Korea: Health Insurance Review & Assessment Service; c2020 [cited 2021 Jan 30]. Available from: http://opendata.hira.or.kr/.
22. Organisation for Economic Co-Operation and Development. Health care resources [Internet]. Paris, France: Organisation for Economic Co-operation and Development; 2018 [cited 2021 Jan 30]. Available from: https://stats.oecd.org/index.aspx?queryid=30183.
23. Lee H, Ugay D, Hong S, Kim Y. Alzheimer's disease diagnosis using misfolding proteins in blood. Dement Neurocogn Disord 2020;19:1–18.



24. Youn YC, Kang S, Suh J, Park YH, Kang MJ, Pyun JM, et al. Blood amyloid-β oligomerization associated with neurodegeneration of Alzheimer's disease. Alzheimers Res Ther 2019;11:40.



25. Dominguez JC, De Guzman MF, Uy JG, Yu JM, Ligsay A, Kang S, et al. P4‐549: Plasma‐based biomarker MDS‐OAβ differentiates Alzheimer's disease from other etiologies. Alzheimers Dement 2019;15(7S Part 29):P1527–P1528.

26. Mattke S, Cho SK, Bittner T, Hlavka J, Hanson M. Blood-based biomarkers for Alzheimer's pathology and the diagnostic process for a disease-modifying treatment: Projecting the impact on the cost and wait times. Alzheimers Dement (Amst) 2020;12:e12081.



28. Lam J, Hlavka J, Mattke S. O1‐11‐02: Estimated impact of diagnostic tools for primary care settings on demand for specialist visits for patients with prodromal Alzheimer's disease. Alzheimers Dement 2019;15(7S Part 4):P224–P225.

- TOOLS