|
 |
- Search
| Ann Geriatr Med Res > Volume 27(4); 2023 > Article |
|
Abstract
Background
The short Dutch Safety Management Screening (DSMS) is applied at hospital admission of all patients aged >70 years to assess vulnerability. Screening of four geriatric domains aims to prevent adverse outcomes and may support targeted discharge planning for post-acute care. We explored whether the DSMS criteria for acutely admitted patients were associated with rehabilitation-oriented care needs.
Methods
This retrospective cohort study included community-dwelling patients aged ≥70 years acutely admitted to a tertiary hospital. We recorded patient demographics, morbidity, functional status, malnutrition, fall risk, and delirium and used descriptive analysis to calculate the risks by comparing the discharge destination groups.
Results
Among 491 hospital discharges, 349 patients (71.1%) returned home, 60 (12.2%) were referred for geriatric rehabilitation, and 82 (16.7%) to other inpatient post-acute care. Non-home referrals increased with age from 21% (70–80 years) to 61% (>90 years). A surgical diagnosis (odds ratio [OR]=4.92; 95% confidence interval [CI], 2.03–11.95), functional decline represented by Katz-activities of daily living positive screening (OR=3.79; 95% CI, 1.76–8.14), and positive fall risk (OR=2.87; 95% CI, 1.31–6.30) were associated with non-home discharge. The Charlson Comorbidity Index did not differ significantly between the groups.
Conclusion
Admission diagnosis and vulnerability screening outcomes were associated with discharge to rehabilitation-oriented care in patients >70 years of age. The usual care data from DSMS vulnerability screening can raise awareness of discharge complexity and provide opportunities to support timely and personalized transitional care.
A growing number of older hospital patients can benefit from rehabilitation-oriented post-acute care (PAC) to improve their functional outcomes after hospital discharge.1,2) However, age is not an identifying criterion for referral for geriatric rehabilitation. Rather, multidisciplinary assessments and geriatric expertise must establish a genuine need for geriatric rehabilitation in older or more vulnerable hospital patients.3,4) These PAC decisions extend across healthcare settings and are professionally and managerially challenging for hospital teams.5-9)
To support PAC decision-making and enhance the coordination of services following discharge from the hospital, discharge planning should preferably start from admission by following candidates for PAC.10-12) Patient characteristics such as older age, female sex, frailty, lower functional or cognitive status at admission, comorbidities, and length of hospital stay are associated with the development of rehabilitation needs and functional impairments during hospital stays.13-15) To prevent functional decline in vulnerable patients and other adverse outcomes such as institutionalization, various vulnerability screening instruments have been developed.16-18) The vulnerability score of the mandatory Dutch Safety Management System (DSMS) was introduced in Dutch hospitals in 2012 and has been applied to all patients aged >70 years at admission. The DSMS tool consists of short screening instruments in four geriatric domains: delirium, functional impairment, malnutrition, and fall risk.19-22)
Early identification of vulnerable older patients at hospital admission aims to diminish the risk of functional decline during the hospital stay through targeted in-hospital geriatric interventions. Subsequently, early and repeated assessments of rehabilitation needs, exploration of individual motivation, and establishment of an individual prognosis for recovery may identify candidates for geriatric rehabilitation early during their hospital stay and enhance personalized PAC decision-making.11,12) Although the mandatory DSMS screening of seniors at hospital admission was not designed nor validated to identify patients to undergo rehabilitation, an association could exist between the “risk of adverse outcome profile” in these patients and the appropriateness of rehabilitation-oriented care at discharge. Early profiling of potential geriatric rehabilitation candidates using available demographic and clinical admission data, including vulnerability scores, may allow for early decision-making concerning rehabilitation-oriented PAC. We hypothesized that DSMS vulnerability scores would differ between patients referred for geriatric rehabilitation and those discharged home. Therefore, we sought to identify patient characteristics related to the DSMS screening domains that were associated with referral to rehabilitation-oriented care after an acute hospital stay.
Amsterdam University Medical Centers is a large (1,700-bed) tertiary academic medical center with two facilities. Both hospitals are situated in an urban health region and provide specialized medical care to a large, predominantly urbanized region. One hospital has a geriatric rehabilitation unit. Skilled nursing facilities, nursing homes, and private care organizations in the area provide rehabilitation-oriented PAC consisting of geriatric rehabilitation and short-stay residential care. Short-stay residential care is indicated when older patients require temporary nursing home care for recovery.23) We undertook a retrospective cohort study of community-dwelling patients aged >70 years who were discharged from the hospital between January 15 and May 15, 2019.
This study included hospital episodes of community-dwelling patients aged >70 years discharged after acute admission from a single facility. Acute admission was defined as an admission following emergency room admission. The minimum hospital stay was one night. If a patient was admitted more than once during the study period, we included the last hospital episode following the acute admission. We excluded admitted patients who had died and those discharged from other hospitals, and included patients discharged to the in-hospital geriatric rehabilitation unit. Three subgroups of patients were formed according to discharge destination: home, geriatric rehabilitation, and other PAC in a nursing home. Usual care data were extracted from the patients’ medical records. The demographic variables included age, sex, place of residence before admission, and discharge disposition (home, nursing home, or other hospital). Data on the living conditions were not available. Clinical data included attending medical specialty; admission diagnosis; comorbidities; and DSMS data on functional status, nutritional status, falling risk, and presence of delirium symptoms. We collected DSMS data within 48 hours of admission and information concerning consultant specialists, paramedical treatment, and length of hospital stay. The discharge destination for inpatient PAC was geriatric rehabilitation or other nursing home care.
Table 1 presents the vulnerability screening system of the DSMS. This system consists of the Simplified Nutritional Assessment Questionnaire (SNAq) for nutritional status, Katz activities of daily living (ADL) for functional status, and screening questions for delirium and falls.24-26) In the population under study, the adapted version of DSMS was used. The falling risk was assessed using the Johns Hopkins Risk of Falls Assessment Tool (JHRFAT) instead of a single question regarding the history of falls. The JHRFAT is widely used for measuring age, fall history, incontinence, medication use, use of patient-care equipment, mobility, and cognition. Scores of 6–13 and >13 points indicate moderate and severe fall risks, respectively.27,28) We used the Delirium Observation Screening Scale (DOS) to identify the confusion symptoms. The DOS comprises 13 items in seven domains (consciousness, attention, thinking, memory/orientation, psychomotor activity, mood, and perception) and is applied to the presence of delirium symptoms instead of three screening questions on the confusion symptoms. Each item of the DOS was scored during one 8-hour nursing shift (day/evening/night). A score of three or more points was considered positive.29,30)
In the DSMS tool, the score of each separate instrument is dichotomized into the presence or absence of risk and summed to obtain the DSMS score for vulnerability, with a range of 0–4. Vulnerability is defined as DSMS scores of ≥3 and ≥1 in patients aged 70–79 and ≥80 years, respectively.17,19) Table 1 lists the components of the DSMS vulnerability score and vulnerability calculation. The age-adjusted Charlson Comorbidity Index (CCI), based on reported comorbidities, adds one point for every decade over 40 years of age.31)
We analyzed the data using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA). According to the discharge destination after the hospital stay, the data were divided into home (H), geriatric rehabilitation (GR), and other nursing home care (NH). Comorbidity data were computed using the age-adjusted CCI.32) When the Katz-ADL or JHFRAT scores were assessed more than once during the hospital stay, we analyzed the final score. Next to DOSs ≥3, the number of positive DOSs (≥3) was used as an additional variable.
Data were analyzed according to the discharge destination (H, GR, and NH). For analysis of total inpatient PAC discharge, the GR and NH groups were combined to form the “non-home group.” We performed comparisons between groups using χ2 tests for nominal data, Kruskal-Wallis tests for ordinal data, and t-tests for normally distributed continuous data. According to the original DSMS screening, the scores of the adapted DSMS were dichotomized into the presence or absence of risk to calculate the vulnerability score. We calculated the odds ratio (OR) with 95% confidence intervals (CI) of the independent variables “age,” “surgical diagnosis,” “age-adjusted CCI,” and the DSMS criteria using logistic regression analysis comparing home and non-home discharge. Bivariate correlations were evaluated (Pearson coefficient). To calculate the OR for age-adjusted CCI, we dichotomized the data according to the median value (6) in our cohort.33,34)
The Medical Ethics Committee of the University Medical Centers Amsterdam reviewed and approved the study protocol (File No. 2018621). Also, this study complied the ethical guidelines for authorship and publishing in the Annals of Geriatric Medicine and Research.35)
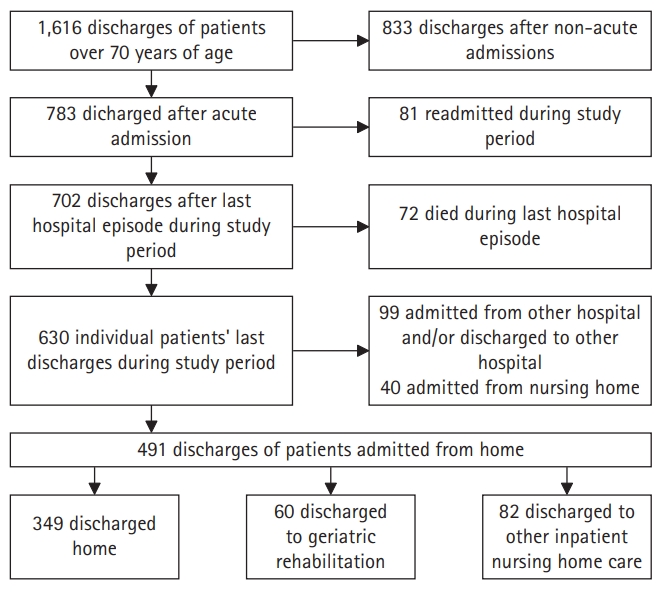
Fig. 1 shows a flow diagram of the study inclusion process. Among 491 total patient records included in this study, 349 (71.1%) patients were discharged H, 60 (12.2%) to GR, and 82 (16.7%) to NH. In the NH group, most (75.6%) were referred for short-stay residential care, recovery care in a nursing home for general medical needs that did not require medical specialist care, or GR.23) A minority of this group (24.4%) was referred for palliative intermediate or long-term care. Supplementary Table S1 provides an overview of the NH group.
Overall, 55.4% of the patients were male. In the H group, 59.3% were men. The sexes were evenly matched in the GH group and were 42.7% in the NH group.
In the 71–80-years age group, 79% were discharged H group, 11% to GR group, and 10% to NH group. In patients >90 years of age, 39% were discharged H group, 23% to GR group, and 38% to NH group. An overview of the data is presented in Table 2.
Among GR patients, 70% were acute orthopedic or trauma patients, in contrast to the H group with 12.6% surgical patients. Internal medical patients comprised 35.5% of the H group, 5.0% of the GR group, and 40.2% of the NH group. Neurological or neurosurgical patients comprised 12.9% of the H-group, 8.3% of the GR group, and 25.7% of the NH group. The mean age-adjusted CCI was 7.18 in the H group, 7.57 in the GR group, and 7.65 in the NH group (p=0.186). Overviews of the comorbidity data and main diagnoses are presented in Supplementary Tables S2 and S3.
DOS scores were missing for 52% of the participants, SNAq scores in 16%, and Katz-ADL in 13%.
The JHFRAT data were complete. Symptoms of delirium (DOS ≥3) were present in 37% of the H patients, 49% of GR patients, 63% of NH patients, and 57% of all non-home discharged patients. Delirium symptoms registered on 2 or more days were present in 6% of H-group patients, 16% of GR patients, 27% of NH, and 22% of all non-home patients. Functional status was low in 28% of patients discharged home compared to 79% of GR patients, 69% of NH patients, and 73% of all non-home discharged patients. A medium or high risk of falling was observed in 52% of participants in the H-group, 73% of the GR group, 82% of the NH group, and 78% of all non-home discharged patients.
DSMS vulnerability scores were present in 30% of H group patients and 70% of NH patients. Vulnerability, according to DSMS scoring was present in 44% of H-group patients, 67% of GR patients, 75% of NH patients, and 72% of all non-home discharged patients. Table 3 presents an overview of the data. The graphs are provided in Supplementary Figs. S1 and S2.
Patients with trauma or acute orthopedic needs (adjusted OR=4.92; 95% CI, 2.03–11.95) had higher odds for non-home discharge. The odds for non-home discharge were highest for patients with functional impairment, as represented by positive Katz-ADL (OR=3.79; 95% CI, 1.76–8.13) and JHFRAT scores on the risk of falling (OR=2.87; 95% CI, 1.31–6.29). We observed no associations between positive DOS (OR=2.12; 95% CI, 0.99–4.55) or SNAq screening (OR=1.64; 95% CI, 0.73–3.70) and non-home discharge. Table 4 presents an overview of the crude and adjusted ORs.
In this cohort of acutely admitted community-dwelling patients, two subscores of the DSMS vulnerability tool were associated with discharge to geriatric rehabilitation or other nursing home care. Usual care data on vulnerability contains valuable information for PAC decision-making. The most distinctive differences between home and non-home hospital discharge were the DSMS subscores for functional status (Katz-ADL) and falling risk (JHFRAT), both of which are multidomain measurement instruments.
Previous studies on the predictive properties of the DSMS vulnerability score have reported contradictory findings regarding early readmission and mortality in older hospital patients.20,21,36) No association was found between DSMS vulnerability and mortality, complications, or readmission in geriatric, cardiac, or gynecological patients.19,37-39) However, in patients with hip fractures, the DSMS vulnerability score was positively associated with mortality and a complicated rehabilitation trajectory.40,41) Moreover, low to moderate prognostic accuracy has been reported for functional decline, morbidity, hospital readmission, institutionalization, and long-term survival.19)
In a cohort of patients discharged from a geriatric ward, positive scores on all four domains of the DSMS vulnerability tool were associated with post-discharge institutionalization; however, the type of PAC was not specified.22) In our cohort of older patients discharged from all hospital wards, we observed a positive association between DSMS vulnerability sub-scores and referral to rehabilitation-oriented PAC The ORs were the highest for positive Katz-ADL (functional domain) and JHFRAT (falling risk) scores. This finding is consistent with evidence that functional metrics are significant predictors of multiple hospital outcomes, including the likelihood of discharge home and the risk of poorer functional status after acute care.42) Functional recovery and safe mobility are important geriatric rehabilitation goals. The application of DSMS screening enhances the awareness of rehabilitation needs, thus targeting potential candidates for geriatric rehabilitation at an early stage.
Most participants in the geriatric rehabilitation group in this study were patients with trauma or acute orthopedic needs and aged >80 years. As in our study, the Dutch hip fracture cohort study found that seniority, premorbid mobility problems, and premorbid Katz-ADL were independent predictors of discharge to geriatric rehabilitation vs. home.43) The original DSMS did not include a separate mobility screening; however, the JHFRAT in the adapted DSMS contains three mobility items: the need for supervision or assistance when walking, unsteady walking, and sensory loss affecting mobility. A positive JHFRAT score in our cohort had positive odds for non-home discharge (adjusted OR=2.87; 95% CI, 1.31–6.29). In the Dutch hip fracture cohort, a higher premorbid Katz-ADL score and a history of dementia distinguished between discharge to a nursing home and discharge home.43) In our study, a DOS of ≥3, which indicated the presence of delirium symptoms, did not show positive odds for non-home discharge from the hospital (OR=2.12; 95% CI, 0.99–4.55). While other studies reported that delirium in patients with hip fractures was an independent predictor of adverse outcomes, our results did not confirm this association.44-46)
In our cohort, a positive DSMS vulnerability score upon hospital admission indicated a certain likelihood of rehabilitation need. Being vulnerable or mildly frail does not imply the absence of rehabilitation potential.4) The identification of future geriatric rehabilitation candidates presents an opportunity to optimize in-hospital geriatric care and personalize PAC decision-making. A positive vulnerability score inspires the exploration of all factors relevant to decision-making. Comprehensive Geriatric Assessment (CGA), multidisciplinary team meetings, and the involvement of patients and families can effectively contribute to patient-centered discharge planning.47) Frailty measures such as the CGA-related frailty index may have prognostic value for rehabilitation outcomes.48,49) This frailty index, as well as the DSMS vulnerability score, can be derived from automated data and facilitates discharge decision-making by allowing the early identification of patients who may later require PAC.50)
We analyzed the data of acutely admitted patients who were discharged from a single tertiary hospital. Both of these factors may have influenced the case mix. We assumed that the discharge of acutely admitted patients was the most representative of our research question because admission to rehabilitation-oriented PCA requires acute functional loss. This restriction and the ongoing reorganization of the two hospitals may have accounted for the change in patient flow, resulting in a high percentage of patients with trauma and a low percentage with neurological conditions in our cohort.
Our dataset has some limitations. First, due to privacy laws, data on living arrangements were not available; although living alone is an influential factor in PAC referral decisions. Second, nearly 50% of the adapted-DSMS screening data for delirium were missing. The DOS score was applied only when confusion was observed at hospital admission. The missing DOS scores explain the low percentage of completed DSMS vulnerability scores. Instructions on the application of this sub-score are important to avoid missing data. The comprehensiveness of both the DOS and JHFRAT may influence the feasibility of the DSMS.
To our knowledge, this is the first Dutch study to address the relationship between routine vulnerability screening at hospital admission and discharge for geriatric rehabilitation. DSMS data are available in the electronic health records of all Dutch hospitals and can be used to identify potential candidates for rehabilitation-oriented PCA. These findings support hospital practices concerning geriatric treatment and facilitate the timely and careful addressing of discharge dilemmas.
As the JHRFAT in the adapted DSMS is a multidimensional “geriatric” instrument used to measure the falling risk, it may have accounted for the higher accuracy of vulnerability measurement compared to the screening question from the original DSMS.
DSMS vulnerability data can be used to predict discharge decisions. Timely PAC decision-making by liaison nurses, geriatricians, or rehabilitation specialists adds to the quality of transitional care. Information on living conditions and family support can further contribute to decision-making.
The inclusion of vulnerability scores in handovers can help to evaluate patient progress during rehabilitation. Frailty status may change during rehabilitation. The ADL status before hospital admission represents a parameter for goal setting in rehabilitation and supports the monitoring of functional gain.
To properly assess the association between vulnerability, appropriateness of referral decisions, and outcomes of rehabilitation-oriented PCA, we recommend a prospective cohort study with follow-up after transfer to a rehabilitation-oriented PAC.
DSMS vulnerability screening with a higher domain score for functional impairment and falling risk indicated higher odds for non-home discharge. Older surgical patients had the highest risk of being transferred to PCA. The usual care data of vulnerability screening at hospital admission can trigger awareness among professionals of the need for rehabilitation-oriented care at discharge, facilitating an early diligent dialogue with older patients and their families regarding preferred treatment and care after hospital discharge.
ACKNOWLEDGMENTS
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4235/agmr.23.0068.
Fig. S1.
DSMS domain scores in non-home versus home discharged group: (A) Katz-ADL, (B) JHFRAT, (C) SNAq, and (D) DOS. DSMS, Dutch Safety Management Screening; Katz-ADL, Katz activities of daily living score (0–6); JHFRAT, Johns Hopkins Falls Risk Assessment Tool; SNAq, Short Nutrition Assessment Questionnaire (0–4); DOS, Delirium Observation Scale score (No DOS >2; 1DOS >2; 2-6DOS >2; >6DOS >2).
Fig. S2.
Distribution of DSMS scores (0–4) in non-home (n=99) and home (n=107) discharged patients. DSMS, Dutch Safety Management Screening.
Table 1.
Original and adapted DSMS vulnerability screening
| Original DSMS screening22) | Adapted DSMS screening | |
|---|---|---|
| Functional status | Katz-ADL≥2 = 1 point | Unchanged |
| Nutritional status | SNAq≥2 = 1 point | Unchanged |
| Falls risk | Q: Did you fall during the last 6 months? | JHRFAT≥6 = 1 point |
| Yes = 1 point | ||
| Delirium | Q: Do you have memory problems (Y/N); did you need help in basic ADL, in the last 24 hours (Y/N); did you previously experience confusion (Y/N) | DOSs≥3 = 1 point |
| ≥1 Yes = 1 point | ||
| DSMS score | 0–4 points | Unchanged |
| Vulnerability | Age<80 and ≥3 points | Unchanged |
| Age≥80 and ≥1 point |
Table 2.
Demographic characteristics, referring specialism and co-morbidity in discharge destination groups
Table 3.
DSMS vulnerability screening of delirium symptoms (DOSs), nutritional (SNAq) and functional (Katz-ADL) status, risk of falls (JHFRAT) in discharge destination groups
Values are presented as number (%).
DSMS, Dutch Safety Management Screening; DOS, Delirium Observation Screening score; SNAq, Short Nutrition Assessment Questionnaire; Katz-ADL, Katz activities of daily living score; JHFRAT, Johns Hopkins Fall Risk Assessment Tool; GR, geriatric rehabilitation; NH, inpatient nursing home care, not geriatric rehabilitation.
Table 4.
Crude and adjusted odds ratios in non-home versus home discharged patients
CCI, Charlson Comorbidity Index; DOS, Delirium Observation Screening score; Katz-ADL, Katz activities of daily living score; JHFRAT, Johns Hopkins Fall Risk Assessment Tool; SNAq, Short Nutrition Assessment Questionnaire; DSMS, Dutch Safety Management Screening; OR, odds ratio; CI, confidence interval.
REFERENCES
1. Grund S, Gordon AL, van Balen R, Bachmann S, Cherubini A, Landi F, et al. European consensus on core principles and future priorities for geriatric rehabilitation: consensus statement. Eur Geriatr Med 2020;11:233–8.



2. van Seben R, Reichardt LA, Aarden JJ, van der Schaaf M, van der Esch M, Engelbert RH, et al. The course of geriatric syndromes in acutely hospitalized older adults: the hospital-ADL study. J Am Med Dir Assoc 2019;20:152–8.


3. Bowles KH, Holmes JH, Ratcliffe SJ, Liberatore M, Nydick R, Naylor MD. Factors identified by experts to support decision making for post acute referral. Nurs Res 2009;58:115–22.



4. de Groot AJ, Wattel EM, van Dam CS, van Balen R, van der Wouden JC, Hertogh CM. Referral to geriatric rehabilitation: a scoping review of triage factors in acutely hospitalised older patients. Age Ageing 2022;51:afac015.



5. Goncalves-Bradley DC, Lannin NA, Clemson L, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev 2022;2:CD000313.


6. Patel H, Yirdaw E, Yu A, Slater L, Perica K, Pierce RG, et al. Improving early discharge using a team-based structure for discharge multidisciplinary rounds. Prof Case Manag 2019;24:83–9.


7. Ubbink DT, Tump E, Koenders JA, Kleiterp S, Goslings JC, Brolmann FE. Which reasons do doctors, nurses, and patients have for hospital discharge?: a mixed-methods study. PLoS One 2014;9:e91333.



8. Bai AD, Dai C, Srivastava S, Smith CA, Gill SS. Risk factors, costs and complications of delayed hospital discharge from internal medicine wards at a Canadian academic medical centre: retrospective cohort study. BMC Health Serv Res 2019;19:935.




9. Cruz-Jentoft AJ, Daragjati J, Fratiglioni L, Maggi S, Mangoni AA, Mattace-Raso F, et al. Using the Multidimensional Prognostic Index (MPI) to improve cost-effectiveness of interventions in multimorbid frail older persons: results and final recommendations from the MPI_AGE European Project. Aging Clin Exp Res 2020;32:861–8.



10. Liu SK, Montgomery J, Yan Y, Mecchella JN, Bartels SJ, Masutani R, et al. Association between hospital admission risk profile score and skilled nursing or acute rehabilitation facility discharges in hospitalized older adults. J Am Geriatr Soc 2016;64:2095–100.




11. Oseran AS, Lage DE, Jernigan MC, Metlay JP, Shah SJ. A “hospital-day-1” model to predict the risk of discharge to a skilled nursing facility. J Am Med Dir Assoc 2019;20:689–95.


12. Stefanacci RG, Sloane PD, Zimmerman S. A crystal ball to aid hospital discharge planning. J Am Med Dir Assoc 2019;20:655–6.


13. Cho JS, Hu Z, Fell N, Heath GW, Qayyum R, Sartipi M. Hospital discharge disposition of stroke patients in Tennessee. South Med J 2017;110:594–600.



14. Condorhuaman-Alvarado PY, Menendez-Colino R, Mauleon-Ladrero C, Diez-Sebastian J, Alarcon T, Gonzalez-Montalvo JI. Predictive factors of functional decline at hospital discharge in elderly patients hospitalised due to acute illness. Rev Esp Geriatr Gerontol 2017;52:253–6.


15. Veronese N, Siri G, Cella A, Daragjati J, Cruz-Jentoft AJ, Polidori MC, et al. Older women are frailer, but less often die then men: a prospective study of older hospitalized people. Maturitas 2019;128:81–6.



16. Carpenter JG, Berry PH, Ersek M. Nursing home care trajectories for older adults following in-hospital palliative care consultation. Geriatr Nurs 2017;38:531–6.



17. Heim N, van Fenema EM, Weverling-Rijnsburger AW, Tuijl JP, Jue P, Oleksik AM, et al. Optimal screening for increased risk for adverse outcomes in hospitalised older adults. Age Ageing 2015;44:239–44.


18. Poh AW, Teo SP. Utility of frailty screening tools in older surgical patients. Ann Geriatr Med Res 2020;24:75–82.




19. Warnier RM, van Rossum E, van Kuijk SM, Magdelijns F, Schols JM, Kempen GI. Frailty screening in hospitalised older adults: how does the brief Dutch National Safety Management Program perform compared to a more extensive approach? J Clin Nurs 2020;29:1064–73.



20. Snijders BM, Emmelot-Vonk MH, Souwer ET, Kaasjager HA, van den Bos F. Prognostic value of screening instrument based on the Dutch national VMS guidelines for older patients in the emergency department. Eur Geriatr Med 2021;12:143–50.



21. van Dam CS, Trappenburg MC, Ter Wee MM, Hoogendijk EO, de Vet HC, Smulders YM, et al. The accuracy of four frequently used frailty instruments for the prediction of adverse health outcomes among older adults at two Dutch emergency departments: findings of the AmsterGEM study. Ann Emerg Med 2021;78:538–48.


22. Oud FM, Wolzak NK, Spies PE, Zaag-Loonen HJV, van Munster BC. The predictive value of the ‘VMS frail older patients’ for adverse outcomes in geriatric inpatients. Arch Gerontol Geriatr 2021;97:104514.


23. van den Besselaar JH, Hartel L, Wammes JD, MacNeil-Vroomen JL, Buurman BM. ‘Patients come with two garbage bags full of problems and we have to sort them.’: a qualitative study of the experiences of healthcare professionals on patients admitted to short-term residential care in the Netherlands. Age Ageing 2021;50:1361–70.



24. Rolland Y, Perrin A, Gardette V, Filhol N, Vellas B. Screening older people at risk of malnutrition or malnourished using the Simplified Nutritional Appetite Questionnaire (SNAQ): a comparison with the Mini-Nutritional Assessment (MNA) tool. J Am Med Dir Assoc 2012;13:31–4.


25. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185:914–9.


26. Gerrard P. The hierarchy of the activities of daily living in the Katz index in residents of skilled nursing facilities. J Geriatr Phys Ther 2013;36:87–91.


27. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for Predicting Falls on Inpatient Medicine Services. J Nurs Care Qual 2017;32:108–13.


28. Kim YJ, Choi KO, Cho SH, Kim SJ. Validity of the Morse Fall Scale and the Johns Hopkins Fall Risk Assessment Tool for fall risk assessment in an acute care setting. J Clin Nurs 2022;31:3584–94.



29. Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract 2003;17:31–50.


30. Park J, Jeong E, Lee J. The delirium observation screening scale: a systematic review and meta-analysis of diagnostic test accuracy. Clin Nurs Res 2021;30:464–73.



31. Bernard S, Inderjeeth C, Raymond W. Higher Charlson Comorbidity Index scores do not influence Functional Independence Measure score gains in older rehabilitation patients. Australas J Ageing 2016;35:236–41.



32. Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am Health Drug Benefits 2019;12:188–97.


33. Bonaventura A, Leale I, Carbone F, Liberale L, Dallegri F, Montecucco F, et al. Pre-surgery age-adjusted Charlson Comorbidity Index is associated with worse outcomes in acute cholecystitis. Dig Liver Dis 2019;51:858–63.


34. Chang CM, Yin WY, Wei CK, Wu CC, Su YC, Yu CH, et al. Adjusted Age-Adjusted Charlson Comorbidity Index Score as a Risk Measure of Perioperative Mortality before Cancer Surgery. PLoS One 2016;11:e0148076.



35. Noh JH, Jung HW, Ga H, Lim JY. Ethical guidelines for publishing in the Annals of Geriatric Medicine and Research. Ann Geriatr Med Res 2022;26:1–3.




36. Schuijt HJ, Oud FM, Bruns EJ, van Duijvendijk P, Van der Zaag-Loonen HJ, Spies PE, et al. Does the Dutch Safety Management Program predict adverse outcomes for older patients in the emergency department? Neth J Med 2020;78:244–50.

37. Oud FM, de Rooij SE, Schuurman T, Duijvelaar KM, van Munster BC. Predictive value of the VMS theme ‘Frail elderly’: delirium, falling and mortality in elderly hospital patients. Ned Tijdschr Geneeskd 2015;159:A8491.

38. Jepma P, Verweij L, Tijssen A, Heymans MW, Flierman I, Latour CH, et al. The performance of the Dutch Safety Management System frailty tool to predict the risk of readmission or mortality in older hospitalised cardiac patients. BMC Geriatr 2021;21:299.




39. van der Zanden V, Paarlberg KM, van der Zaag-Loonen HJ, Meijer WJ, Mourits MJ, van Munster BC. Risk assessment for postoperative outcomes in a mixed hospitalized gynecological population by the Dutch safety management system (Veiligheidsmanagementsysteem, VMS) screening tool ‘frail elderly’. Arch Gynecol Obstet 2021;304:465–73.




40. Folbert EC, Hegeman JH, Gierveld R, van Netten JJ, Velde DV, Ten Duis HJ, et al. Complications during hospitalization and risk factors in elderly patients with hip fracture following integrated orthogeriatric treatment. Arch Orthop Trauma Surg 2017;137:507–15.



41. Winters AM, Hartog LC, Roijen H, Brohet RM, Kamper AM. Relationship between clinical outcomes and Dutch frailty score among elderly patients who underwent surgery for hip fracture. Clin Interv Aging 2018;13:2481–6.




42. So C, Lage DE, Slocum CS, Zafonte RD, Schneider JC. Utility of functional metrics assessed during acute care on hospital outcomes: a systematic review. PM R 2019;11:522–32.




43. van Dartel D, Vermeer M, Folbert EC, Arends AJ, Vollenbroek-Hutten MM, Hegeman JH, et al. Early predictors for discharge to geriatric rehabilitation after hip fracture treatment of older patients. J Am Med Dir Assoc 2021;22:2454–60.


44. Low S, Wee E, Dorevitch M. Impact of place of residence, frailty and other factors on rehabilitation outcomes post hip fracture. Age Ageing 2021;50:423–30.



45. Lisk R, Yeong K, Enwere P, Jenkinson J, Robin J, Irvin-Sellers M, et al. Associations of 4AT with mobility, length of stay and mortality in hospital and discharge destination among patients admitted with hip fractures. Age Ageing 2020;49:411–7.



46. Dolan MM, Hawkes WG, Zimmerman SI, Morrison RS, Gruber-Baldini AL, Hebel JR, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci 2000;55:M527–34.


47. Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017;9:CD006211.


48. Arjunan A, Peel NM, Hubbard RE. Feasibility and validity of frailty measurement in geriatric rehabilitation. Australas J Ageing 2018;37:144–6.