|
 |
- Search
| Ann Geriatr Med Res > Volume 27(3); 2023 > Article |
|
Abstract
Background
In this study, we aimed to examine the changes in delirium during hospitalization of patients and its association with behavioral and psychological symptoms of dementia (BPSD), as well as improvements in activities of daily living (ADL).
Methods
A longitudinal, retrospective cohort study was conducted involving 83 older adults (≥65 years) with hip fractures. We collected Mini-Mental State Examination (MMSE) and Functional Independence Measure-motor domain (m-FIM) assessment results from the medical charts at two time points: baseline (first week of hospitalization) and pre-discharge (final week before discharge). Additionally, we collected data on delirium and BPSD at three points: baseline, week 2 post-admission, and pre-discharge. We performed univariate logistic regression analysis using changes in m-FIM scores as the dependent variable and MMSE and m-FIM scores at baseline and pre-discharge, along with delirium and BPSD subtypes at baseline, week 2 post-admission, and pre-discharge, as the explanatory variables. Finally, we performed a multivariate logistic regression analysis incorporating the significant variables from the univariate analysis to identify factors associated with ADL improvement during hospitalization.
Results
We observed significant correlations between ADL improvement during hospitalization and baseline m-FIM and MMSE scores, hypoactive delirium state, and BPSD subtype pre-discharge. Notably, all participants with hypoactive symptoms before discharge exhibited some subtype of delirium and BPSD at baseline.
The incidence of hip fractures among older adults is increasing in many countries,1) including Japan.2) Falls are a common cause of hip fracture3) and are associated with dementia and a decline in cognitive function.4) A recent longitudinal study reported that even at 12 months post-hip fracture, activities of daily living (ADL) scores failed to recover to pre-fracture levels, with almost 60% of patients experiencing at least one ADL functional limitation.5) These findings suggest the importance of identifying factors that influence the improvement in ADL. In the rehabilitation of older adult patients recovering hip fractures, research has demonstrated the importance of addressing delirium and behavioral and psychological symptoms of dementia (BPSD) arising from cognitive impairment during hospitalization.6,7)
Delirium and BPSD during hospitalization hinder improvements in ADL.8-10) Marcantonio et al.9) reported that patients with delirium at admission may experience deterioration of their mental state, and Gialanella et al.10) reported that BPSD at admission hinders engagement in rehabilitation, potentially hindering ADL improvement during hospitalization among older adults with hip fractures. However, no longitudinal studies have investigated the subtypes of delirium and BPSD that hinder ADL improvement at specific time points.
Delirium and BPSD can be divided into several subtypes based on symptoms, such as hallucinations and delusions, disturbing speech, excitatory behavior, and an altered sleep-wake cycle11); it can generally be categorized into hyperactive, hypoactive, and mixed subtypes.12) Moreover, these subtypes can change during hospitalization, suggesting the need to study these subtypes over time. However, most studies investigating the factors associated with ADL improvement among patients with hip fracture during hospitalization13-15) have categorized delirium and BPSD as simply “present” or “not present” and few studies have investigated the subtypes. Furthermore, existing studies have only focused on assessing the status upon admission, but no studies have assessed chronological changes in delirium and BPSD.10,16,17)
Therefore, to clarify the association of delirium and BPSD with ADL improvement, we investigated the changes in delirium and BPSD subtypes throughout the hospitalization period among older adult patients with hip fractures. By elucidating the association between ADL improvement and the timing and onset of subtypes of delirium and BPSD in patients with hip fractures, our findings study could offer valuable insights and important directions and implications for the assessment of delirium and BPSD during hospitalization at general hospitals, guiding appropriate care at each stage of hospitalization.
We conducted a retrospective cohort study using longitudinal data in accordance with the STROBE guidelines.18) This study was approved by the Hiroshima University’s Ethics Review Committee for Life Science and Medical Research with Human Participants (No. Epidemiology 3972) and Kaneda Hospital, Okayama, Japan.
Also, this study complied the ethical guidelines for authorship and publishing in the Annals of Geriatric Medicine and Research.19)
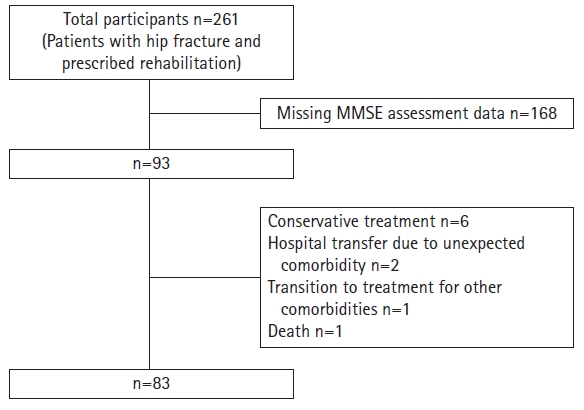
The inclusion criteria for participating in this study were patients who were aged ≥65 years underwent hip fracture surgery and were prescribed rehabilitation at a Japanese general hospital between September 2016 and October 2022. We excluded patients who (1) did not undergo cognitive function assessment, (2) received conservative treatment, (3) were transferred to another hospital or transitioned to treatment for comorbidities, or (4) died.
Among the 261 patients, 93 underwent cognitive function assessments. The remaining 168 patients either did not have adequate cognitive function to undergo assessment or did not consent to participate in the study. Of the 93 remaining patients, six received conservative treatments due to contraindications to surgery, three were transferred to another hospital or transitioned to treatment because of complications, and one patient died. Consequently, our final analysis included 83 patients (Fig. 1).
Following hip fracture surgery, all patients were prescribed physical therapy as a part of rehabilitation plan. Additionally, we prescribed occupational and speech therapies based on each patient’s comorbidities, physical function, and cognitive function, with the goal of maintaining or improving physical and cognitive capabilities.
We assessed the basic characteristics, cognitive function, delirium, BPSD, and ADL of all 83 patients using data from their medical charts. To assess cognitive function, we utilized the results of their Mini-Mental State Examination (MMSE), and for evaluating ADL, we utilized the motor domain scores of the Functional Independence Measures (m-FIM), which served as our outcome variable.
We defined the first week of hospitalization (beginning at admission) as the baseline, the second week of hospitalization (from the end of week 1 to the end of week 2) as week 2 post-admission, and the week before discharge (ending at discharge) as pre-discharge. Data on the types of medications, MMSE scores, and m-FIM scores were collected at two points: at baseline and pre-discharge. Delirium and BPSD results were collected at three time points: baseline, week 2 post-admission, and pre-discharge. As delirium is classified into cases based on whether symptoms resolved within 1 week or persisted for a longer period, cases in which delirium persists for at least 1 week are considered to be more severe.20) Therefore, we assessed delirium and BPSD results at week 2 post-admission in addition to baseline and pre-discharge.
We collected the following patient characteristics: age, sex, length of stay, type of surgery (arthroplasty, internal fixation), comorbidities (orthopedic disease, heart disease, lung disease, mental illness, neurodegenerative disease, and cancer), dementia diagnosis and type (Alzheimer disease, Lewy body dementia), pre-fracture walking ability (independent: yes/no), and types of medications (anti-psychotic drugs, anti-dementia drugs, and anti-anxiety drugs).
We collected data from MMSEs for the assessment of cognitive function.21) The MMSE is a simple assessment tool with established reliability and validity. The maximum score is 30 points, with scores ≤23 points indicating dementia22) and lower scores indicating more severe cognitive impairment. These assessments were performed by occupational or speech therapists.
Delirium and BPSD are typically observed in older adults with dementia. Although they are classified differently,23) their clinical symptoms are similar. Thus, it is difficult to differentiate between these conditions after disease onset.11) Therefore, we considered these two conditions as one. Delirium is classified into three subtypes, including hyperactive, hypoactive, and mixed,24) whereas BPSD is classified into 12 subtypes:delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy, disinhibition, irritability/lability, aberrant motor behavior, nighttime behavior disturbances, and appetite and eating abnormalities.25) A previous study classifying BPSD using cluster analysis reported that it can be clinically classified as hyperactive or hypoactive.26) We identified data on patients’ speech and behavior corresponding to delirium and BPSD from their medical charts using these classifications.24-26) We then categorized these into the following subtypes: hyperactive, hypoactive, and mixed.
For the hyperactive subtype, we considered delirium symptoms including abnormal verbal output, hyperalertness, irritability, euphoria, and combativeness, along with BPSD symptoms including delusions, hallucinations, agitation/aggression, elation/euphoria, disinhibition, irritability/lability, aberrant motor behavior, nighttime behavior disturbances, and appetite and eating abnormalities. Patients with any of these symptoms were classified as hyperactive. For the hypoactive subtype, we considered delirium symptoms including apathy, decreased alertness, withdrawal, and hypersomnolence, along with BPSD symptoms including apathy, depression/dysphoria, and anxiety. Patients exhibiting any of these symptoms were classified as hypoactive. The mixed subtype was defined as a combination of symptoms from hyperactive and hypoactive subtypes. Specifically, this subtype included cases in which the patient exhibited both hyperactive and hypoactive symptoms, such as agitation followed by apathy, during a single assessment period.
The FIM is used to evaluate the extent of an individual’s ADL care needs. In this study, we used the assessment results for the motor domain (m-FIM).27) The m-FIM comprises self-care, sphincter control, and transfers. The m-FIM is known for its high reliability, sensitivity to changes in the functional status of patients undergoing rehabilitation, and ease of implementation.28-30) Each item is assessed on a 7-point scale, ranging from requiring total assistance to being completely independent. The total scores range from 13 to 91 points, with a lower score indicating lower ADL independence. These assessments were performed by physiotherapists.
We calculated the mean, standard deviation, and percentage based on the descriptive statistics of the patient characteristics and assessment results. Statistical analyses were performed using R software (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria). All tests were performed at a significance level of 0.05.
First, we calculated the median change in the m-FIM scores. Subsequently, we categorized the patients into high and low groups based on the changes in their ADL scores, with those at or above the median categorized into the high group and those below the median categorized into the low group.
Thereafter, using a univariate logistic regression model, we analyzed the factors associated with changes in m-FIM scores. The high or low group change in m-FIM scores was set as the outcome variable, whereas the explanatory variables encompassed basic characteristics, types of medications (baseline and pre-discharge), MMSE scores (baseline and pre-discharge), m-FIM scores (baseline and pre-discharge), and delirium and BPSD subtypes (baseline, week 2 post-admission, and pre-discharge).
Finally, we performed multivariate logistic regression analysis to organize and investigate the multiple factors associated with changes in m-FIM. The high or low group change in m-FIM scores was set as the outcome variable, whereas the explanatory variables comprised those with significant associations in the univariate logistic regression analysis. Notably, we observed multicollinearity for m-FIM scores, MMSE scores, and types of medications; therefore, only the baseline values were used as explanatory variables for these items. We confirmed the goodness of fit of the explanatory variables to the outcome variable using the Hosmer–Lemeshow test.
Table 1 presents the patients’ basic characteristics. In this study, 72 patients (86.7%) were women, 63 (75.9%) had comorbidities, 69 (83.1%) had not been diagnosed with dementia, and 38 (45.8%) could walk independently before admission. The mean age was 89.1±7.2 years, the mean length of stay was 51.9±19.8 days, and the mean MMSE score at baseline was 15.5±8.4 points. Among the patients, 73 (88.0%) exhibited delirium or BPSD at least one of the three data collection points.
The median change in the m-FIM score was 27. Thus, the high (n=41) and low (n=42) groups comprised patients with changes in their m-FIM scores of ≥27 points and ≤26 points, respectively. The univariate analysis, performed to identify factors associated with the change in m-FIM scores, revealed significant associations with age (odds ratio [OR]=0.94; 95% confidence interval [CI], 0.87–9.97), independent walking before admission (OR=3.17; 95% CI, 1.31–7.99), anti-psychotic type medications (OR=0.34; 95% CI, 0.19–0.59), m-FIM scores (OR=1.07; 95% CI, 1.01–1.15), MMSE scores (OR=1.27; 95% CI, 1.16–1.42), and pre-discharge hypoactive symptomology (OR=0.17; 95% CI, 0.03–0.57) (Table 2).
The results of the multivariate analysis revealed significant associations among low baseline m-FIM score (OR=0.86; 95% CI, 0.77–0.95), high baseline MMSE score (OR=1.41; 95% CI, 1.22–1.71), and pre-discharge hypoactive symptomology (OR=0.07; 95% CI, 0.01–0.43) (Table 3). The result of the Hosmer–Lemeshow test was not significant (X2(8)=0.64).
In this study, we classified 16 patients as having the hypoactive subtype of delirium and BPSD pre-discharge. We confirmed the subtypes that these participants exhibited at baseline and week 2 post-admission, as shown in Fig. 2. All 16 patients exhibited some form of delirium and BPSD at baseline. However, four patients (25%) did not show delirium or BPSD at week 2 post-admission. Furthermore, eight (50%) and six (37.5%) patients exhibited hyperactive symptomology at baseline and week 2 post-admission, respectively. Finally, two participants (12.5%) exhibited hypoactive symptomology at baseline and three (18.8%) exhibited hypoactive symptomology at week 2 post-admission.
Our investigation of the factors associated with ADL improvement during hospitalization among older adult patients with hip fractures revealed better improvement among patients with higher cognitive function at admission and non-hypoactive delirium and BPSD symptomology before discharge than those among patients without these symptomologies. We also observed that the lower a patient’s ADL independence at admission, the greater the improvement in ADL.
Our results indicated that ADL improved during hospitalization among patients with higher MMSE scores at admission. One explanation for this finding may be that patient cooperation with rehabilitation following hip fracture surgery is easier to achieve when cognitive function is better at admission. Thus, patients with better cognitive function at admission may have been open to future ADL improvement and proactive engagement in rehabilitation, even with the presence of postoperative pain and physical dysfunction. Regarding the association between rehabilitation and ADL improvement, Lenze et al.31) reported that executive dysfunction and apathy caused by cognitive impairment may impede engagement in rehabilitation and act as barriers to ADL improvement. Similarly, Kang et al.32) observed that higher MMSE scores are positively associated with improved walking ability. Although we did not investigate patients’ engagement in rehabilitation, we conjecture that ADL improvement was better among patients with stronger cognitive function at admission as they were proactively engaged in rehabilitation.
Furthermore, our results demonstrated that ADL improvement was more difficult in participants who had hypoactive symptoms as discharge approached. In a study conducted in an acute-phase hospital with similar results, Lenze et al.33) reported reduced ADL improvement in patients with apathy and hypoactive symptoms before discharge, suggesting that a hypoactive state before discharge may impede engagement in rehabilitation and negatively impact ADL improvement. However, Gialanella et al.16) reported that hyperactive symptoms at admission may impede ADL improvement. Although, in our study, a hyperactive state at admission was not identified as a barrier to ADL improvement, further research on the association between the timing of the onset of hyperactive symptoms and ADL is required.
Patients in a hypoactive state are unlikely to regularly exhibit this behavior in everyday context. Thus, they are often misdiagnosed by hospital staff as being “well-behaved” with no symptomatic behaviors.34) However, if hospital staffs do not diagnose a patient’s hypoactivity, ADL improvement may be impeded by the patient’s decreased motivation for rehabilitation and ADL, ultimately reducing opportunities for their participation.35) Thus, hospital staffs must pay close attention to changes in patients’ hypoactivity not only after admission and surgery but also on a daily basis to ensure that they do not overlook hypoactive symptoms during hospitalization. Moreover, providing rehabilitation and everyday care is particularly important to prevent the development of hypoactive symptoms before discharge.
Finally, our results demonstrated that patients with lower ADL independence at admission (i.e., lower FIM scores) exhibited better ADL improvement. Previous studies have reported similar results suggesting that the lower a patient’s FIM score at admission, the greater is the improvement.36) When the FIM score was high at the time of admission, any subsequent improvement in their ADL might have been constrained, due to limitation for further increase in the maximum FIM score. Since the FIM includes tasks with a relatively low difficulty level, the possibility of a ceiling effect cannot be ruled out. This may explain the lack of association between high ADL independence upon admission and ADL improvement in our study.
Considering that most patients in a hypoactive state with subtype symptoms of delirium and BPSD during the study period were in a hyperactive state at admission, only a few remained hypoactive throughout the hospitalization period. Hospitalization phase for these patients included the acute stage after surgery, in which patients experienced a combination of physical factors, such as limited movement; psychological factors including interaction with unfamiliar hospital staff; and environmental factors that differ from their ordinary lives, such as the presence of intravenous drips. This combination of factors may lead them to transition into a hyperactive state. Furthermore, studies have indicated that the use of pharmacotherapy to treat hyperactive patients upon admission may lead to a hypoactive state.37-39) Similarly, based on the number of anti-psychotic drugs used at admission and week 2 after admission compared to pre-discharge, we conjecture that hypoactivity induced by pharmacotherapy cannot be ruled out. Considering these findings, hospital staffs may contribute to ADL improvement during hospitalization through careful multidisciplinary observation and communication of any changes in the patient’s delirium and BPSD subtypes after the first 2 weeks of hospitalization and address these symptoms primarily through non-pharmacological methods.37,40)
This study had several limitations. First, due to the retrospective nature of our delirium and BPSD assessments, we could not perform detailed quantitative evaluation of subtype categories. Second, we could not perform a follow-up on pre-discharge hypoactive symptoms, as a factor impeding ADL improvement beyond the first 2 weeks after admission, preventing us from determining the timing and persistence of the hypoactive state until pre-discharge. Third, we could not consider the impact of physical functioning, such as muscle strength, on ADL improvement. Future prospective longitudinal studies using qualitative assessments of delirium and BPSD along with extended follow ups beyond the second week of hospitalization are necessary. Moreover, future studies are required to examine whether subtypes continue to change after discharge, and if so, how hospital care may influence such changes as well as their impact on ADL post-discharge.
Despite these limitations, the results of our investigation of the change in delirium and BPSD subtypes over time during hospitalization among older patients with hip fractures demonstrated that ADL improvement may be impeded in patients with hypoactive pre-discharge delirium and BPSD symptoms. Previous studies have demonstrated that delirium and BPSD at admission can hinder ADL improvement. However, only a few studies have investigated the chronological changes and subtypes of delirium and BPSD that can hinder ADL improvement during hospitalization. The significance of this study lies in elucidating the potential negative effects of pre-discharge hypoactivity on ADL improvement. These results have implications for patient care in clinical practice. They further underscore the importance of continuous monitoring of changes in delirium and BPSD subtypes throughout hospitalization and preventing any shift to hypoactivity to effectively improve ADL outcomes.
ACKNOWLEDGMENTS
Fig. 2.
The changes in the subtypes of the 16 pre-discharge hypoactive participants at baseline and week 2 post-admission. BPSD, behavioral and psychological symptoms of dementia.
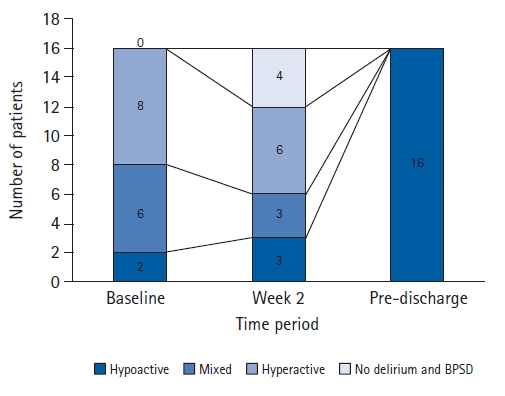
Table 1.
Demographic and characteristics data (n=83)
| Characteristic | Value |
|---|---|
| Age (y) | 89.1±7.2 |
| Sex | |
| Male | 11 (13.2) |
| Female | 72 (86.7) |
| Length of stay (day) | 52.0±19.8 |
| Type of surgery | 83 (100) |
| Arthroplasty | 28 (33.7) |
| Internal fixation | 55 (66.3) |
| Comorbidity | 63 (75.9) |
| Orthopedic disease | 14 (16.9) |
| Heart disease | 19 (22.9) |
| Lung disease | 8 (9.6) |
| Mental illness | 15 (18.1) |
| Neurodegenerative disease | 4 (4.8) |
| Cancer | 3 (3.6) |
| Type of dementia | 14 (16.9) |
| No dementia | 69 (83.1) |
| Alzheimer’s disease | 13 (15.6) |
| Lewy body dementia | 1 (1.2) |
| Pre-fracture walking ability | |
| Independent | 38 (45.8) |
| Number of drugs for anti-psychotic | |
| Baseline | 46 (55.4) |
| Pre-discharge | 36 (43.3) |
| Number of drugs for anti-dementia | |
| Baseline | 7 (8.4) |
| Pre-discharge | 5 (6.0) |
| Number of drugs for anti-anxiety | |
| Baseline | 18 (21.7) |
| Pre-discharge | 18 (21.7) |
| FIM motor score (13–91) | |
| Baseline | 20.5±9.1 |
| Pre-discharge | 50.4±27.0 |
| MMSE total score (0–30) | |
| Baseline | 15.5±8.4 |
| Pre-discharge | 16.3±8.3 |
| Delirium and BPSD | 60 (72.3) |
| Hyperactive | |
| Baselinea) | 42 (50.6) |
| Week 2b) | 33 (39.6) |
| Pre-dischargec) | 33 (39.6) |
| Hypoactive | 28 (33.7) |
| Baselinea) | 7 (8.4) |
| Week 2b) | 12 (14.5) |
| Pre-dischargec) | 16 (19.3) |
| Mixed | 33 (39.8) |
| Baselinea) | 19 (22.9) |
| Week 2b) | 14 (16.9) |
| Pre-dischargec) | 8 (9.6) |
Table 2.
Univariate logistic regression for each variable
| Variable |
m-FIM gain |
p-value | |
|---|---|---|---|
| Low group | High group | ||
| Age (y) | 90.8±6.5 | 87.6±7.7 | 0.047* |
| Sex | |||
| Male | 7 (8.4) | 4 (4.8) | 0.320 |
| Female | 34 (40.9) | 38 (45.8) | - |
| Length of stay (day) | 49.0±22.8 | 54.9±16.2 | 0.180 |
| Type of surgery | |||
| Arthroplasty | 13 (15.7) | 15 (18.1) | - |
| Internal fixation | 28 (33.7) | 27 (32.5) | 0.700 |
| Comorbidity | |||
| Orthopedic disease | 6 (7.2) | 8 (9.6) | 0.590 |
| Heart disease | 7 (8.4) | 12 (14.5) | 0.220 |
| Lung disease | 2 (2.4) | 6 (7.2) | 0.170 |
| Mental illness | 10 (12.0) | 5 (6.0) | 0.150 |
| Neurodegenerative disease | 2 (2.4) | 2 (2.4) | 0.980 |
| Cancer | - | 3 (3.6) | 0.990 |
| Types of dementia | |||
| Alzheimer’s disease | 9 (10.8) | 4 (4.8) | 0.130 |
| Lewy body dementia | 1 (1.2) | - | 0.990 |
| Pre-fracture walking ability | |||
| Independent | 13 (15.7) | 25 (30.1) | 0.010* |
| Number of drugs for anti-psychotic | |||
| Baseline | 31 (37.3) | 15 (18.1) | <0.001* |
| Pre-discharge | 28 (33.7) | 8 (9.6) | <0.001* |
| Number of drugs for anti-dementia | |||
| Baseline | 5 (6.0) | 2 (2.4) | 0.210 |
| Pre-discharge | 3 (3.6) | 2 (2.4) | 0.460 |
| Number of drugs for anti-anxiety | |||
| Baseline | 7 (8.4) | 11 (13.3) | 0.200 |
| Pre-discharge | 7 (8.4) | 11 (13.3) | 0.200 |
| FIM motor score (13–91) | |||
| Baseline | 18.3±9.9 | 22.5±7.8 | 0.046* |
| Pre-discharge | 27.0±14.3 | 73.2±13.0 | <0.001* |
| MMSE total score (0–30) | |||
| Baseline | 10.0±7.2 | 20.8±5.7 | <0.001* |
| Pre-discharge | 11.3±7.5 | 21.2±5.7 | <0.001* |
| Delirium and BPSD | |||
| Hyperactive | |||
| Baselinea) | 25 (59.5) | 17 (40.5) | 0.060 |
| Week 2b) | 20 (60.6) | 13 (39.4) | 0.100 |
| Pre-dischargec) | 17 (51.5) | 16 (48.5) | 0.750 |
| Hypoactive | |||
| Baselinea) | 4 (57.1) | 3 (42.9) | 0.670 |
| Week 2b) | 7 (58.3) | 5 (41.7) | 0.510 |
| Pre-dischargec) | 13 (81.3) | 3 (18.8) | 0.009* |
| Mixed | |||
| Baselinea) | 11 (57.9) | 8 (42.1) | 0.400 |
| Week 2b) | 9 (64.3) | 5 (35.7) | 0.230 |
| Pre-dischargec) | 7 (87.5) | 1 (12.5) | 0.051 |
Table 3.
Multivariate logistic regression
| Variable | Estimate | SE | OR (95% CI) | p-value |
|---|---|---|---|---|
| Age | -0.04 | 0.06 | 0.96 (0.85–1.08) | 0.490 |
| FIM motor score | -0.15 | 0.05 | 0.86 (0.77–0.95) | 0.003* |
| Drugs for anti-psychotic | -0.58 | 0.45 | 0.56 (0.22–1.36) | 0.200 |
| Walking ability | 1.06 | 0.74 | 2.89 (0.68–13.27) | 0.150 |
| MMSE total score | 0.34 | 0.09 | 1.41 (1.22–1.71) | <0.001* |
| Hypoactive | -2.66 | 1.02 | 0.07 (0.01–0.43) | 0.008* |
FIM motor score, drugs for anti-psychotic, and MMSE score are mentioned at the baseline level. Walking ability was recorded as pre-fracture (independent: yes/no); hypoactive status was recorded pre-discharge.
FIM, Functional Independent Measure motor score; MMSE, Mini-Mental State Examination; SE, standard error; OR, odds ratio; CI, confidence interval.
REFERENCES
1. Dhanwal DK, Dennison EM, Harvey NC, Cooper C. Epidemiology of hip fracture: worldwide geographic variation. Indian J Orthop 2011;45:15–22.




2. Orimo H, Yaegashi Y, Hosoi T, Fukushima Y, Onoda T, Hashimoto T, et al. Hip fracture incidence in Japan: Estimates of new patients in 2012 and 25-year trends. Osteoporos Int 2016;27:1777–84.




4. Friedman SM, Menzies IB, Bukata SV, Mendelson DA, Kates SL. Dementia and hip fractures: development of a pathogenic framework for understanding and studying risk. Geriatr Orthop Surg Rehabil 2010;1:52–62.




5. Abeygunasekara T, Lekamwasam S, Lenora J, Alwis G. Quality of life and functional independence of hip fracture patients: data from a single center follow-up study in Sri Lanka. Ann Geriatr Med Res 2021;25:98–104.




6. Carrillo CB, Barr C, George S. Cognitive status and outcomes of older people in orthopedic rehabilitation? A retrospective-cohort study. Geriatrics (Basel) 2020;5:14.



8. Heruti RJ, Lusky A, Barell V, Ohry A, Adunsky A. Cognitive status at admission: does it affect the rehabilitation outcome of elderly patients with hip fracture? Arch Phys Med Rehabil 1999;80:432–6.


9. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc 2000;48:618–24.


10. Gialanella B, Prometti P, Monguzzi V, Ferlucci C. Neuropsychiatric symptoms and rehabilitation outcomes in patients with hip fracture. Am J Phys Med Rehabil 2014;93:562–9.


11. Tanaka T. Factors predicting perioperative delirium and acute exacerbation of behavioral and psychological symptoms of dementia based on admission data in elderly patients with proximal femoral fracture: a retrospective study. Geriatr Gerontol Int 2016;16:821–8.


13. Shibasaki K, Asahi T, Mizobuchi K, Akishita M, Ogawa S. Rehabilitation strategy for hip fracture, focused on behavioral psychological symptoms of dementia for older people with cognitive impairment: a nationwide Japan rehabilitation database. PLoS One 2018;13:e0200143.



14. Heyman N, Nili F, Shahory R, Seleznev I, Ben Natan M. Prevalence of delirium in geriatric rehabilitation in Israel and its influence on rehabilitation outcomes in patients with hip fractures. Int J Rehabil Res 2015;38:233–7.


15. Adunsky A, Levy R, Heim M, Mizrahi E, Arad M. The unfavorable nature of preoperative delirium in elderly hip fractured patients. Arch Gerontol Geriatr 2003;36:67–74.


16. Gialanella B, Ferlucci C, Monguzzi V, Prometti P. Determinants of functional outcome in hip fracture patients: the role of specific neuropsychiatric symptoms. Disabil Rehabil 2015;37:517–22.


17. Gialanella B, Ferlucci C, Monguzzi V, Prometti P. Determinants of outcome in hip fracture: role of daily living activities. Eur J Phys Rehabil Med 2015;51:253–60.

18. Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297.



19. Noh JH, Jung HW, Ga H, Lim JY. Ethical guidelines for publishing in the Annals of Geriatric Medicine and Research. Ann Geriatr Med Res 2022;26:1–3.




20. Wada Y, Yamaguchi N. Delirium in the elderly: relationship of clinical symptoms to outcome. Dementia 1993;4:113–6.



21. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.

22. Fayers PM, Hjermstad MJ, Ranhoff AH, Kaasa S, Skogstad L, Klepstad P, et al. Which mini-mental state exam items can be used to screen for delirium and cognitive impairment? J Pain Symptom Manage 2005;30:41–50.


23. Hessler JB, Schaufele M, Hendlmeier I, Junge MN, Leonhardt S, Weber J, et al. Behavioural and psychological symptoms in general hospital patients with dementia, distress for nursing staff and complications in care: results of the General Hospital Study. Epidemiol Psychiatr Sci 2018;27:278–87.


24. Meagher DJ, Moran M, Raju B, Gibbons D, Donnelly S, Saunders J, et al. Motor symptoms in 100 patients with delirium versus control subjects: comparison of subtyping methods. Psychosomatics 2008;49:300–8.


25. Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997;48:S10–6.

26. Miyagawa A, Kunii Y, Gotoh D, Hoshino H, Kakamu T, Hidaka T, et al. Effects of the Great East Japan Earthquake and the Fukushima Daiichi Nuclear Power Plant accident on behavioural and psychological symptoms of dementia among patients. Psychogeriatrics 2021;21:709–15.




27. Kidd D, Stewart G, Baldry J, Johnson J, Rossiter D, Petruckevitch A, et al. The Functional Independence Measure: a comparative validity and reliability study. Disabil Rehabil 1995;17:10–4.


28. Hetherington H, Earlam RJ, Kirk CJ. The disability status of injured patients measured by the functional independence measure (FIM) and their use of rehabilitation services. Injury 1995;26:97–101.


29. Ottenbacher KJ, Mann WC, Granger CV, Tomita M, Hurren D, Charvat B. Inter-rater agreement and stability of functional assessment in the community-based elderly. Arch Phys Med Rehabil 1994;75:1297–301.


30. Granger CV, Hamilton BB. The Uniform Data System for Medical Rehabilitation report of first admissions for 1992. Am J Phys Med Rehabil 1994;73:51–5.


31. Lenze EJ, Munin MC, Dew MA, Rogers JC, Seligman K, Mulsant BH, et al. Adverse effects of depression and cognitive impairment on rehabilitation participation and recovery from hip fracture. Int J Geriatr Psychiatry 2004;19:472–8.


32. Kang JH, Lee G, Kim KE, Lee YK, Lim JY. Determinants of functional outcomes using clinical pathways for rehabilitation after hip fracture surgery. Ann Geriatr Med Res 2018;22:26–32.




33. Lenze EJ, Munin MC, Dew MA, Marin RS, Butters MA, Skidmore ER, et al. Apathy after hip fracture: a potential target for intervention to improve functional outcomes. J Neuropsychiatry Clin Neurosci 2009;21:271–8.



34. Rigney TS. Delirium in the hospitalized elder and recommendations for practice. Geriatr Nurs 2006;27:151–7.


35. Resnick B, Zimmerman SI, Magaziner J, Adelman A. Use of the Apathy Evaluation Scale as a measure of motivation in elderly people. Rehabil Nurs 1998;23:141–7.


36. Ogawa T, Koike M. Independent factors that attenuate the effectiveness of fracture rehabilitation in improving activities of daily living in female patients aged 80 years and above. Aging Clin Exp Res 2022;34:793–800.



37. van Velthuijsen EL, Zwakhalen SM, Mulder WJ, Verhey FR, Kempen GI. Detection and management of hyperactive and hypoactive delirium in older patients during hospitalization: a retrospective cohort study evaluating daily practice. Int J Geriatr Psychiatry 2018;33:1521–9.



38. Leentjens AF, Molag ML, Van Munster BC, De Rooij SE, Luijendijk HJ, Vochteloo AJ, et al. Changing perspectives on delirium care: the new Dutch guideline on delirium. J Psychosom Res 2014;77:240–1.