|
 |
- Search
| Ann Geriatr Med Res > Volume 27(3); 2023 > Article |
|
Abstract
Background
The role of vitamin D in reducing the risk of falls in older adults has not been clearly demonstrated. This study examined the effectiveness of vitamin D supplementation in reducing the risk of falls in older adults.
Methods
Four databases (Cochrane Library, Embase, PubMed, and CINAHL) were searched without language restrictions or time limitations. These articles were comprehensively screened using EndNote version 20.1 software. A manual search of the reference lists of the identified studies was also performed. The analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta–Analyses (PRISMA) guidelines. The pooled evidence was analyzed using RevMan software version 5.4.
Results
Seventeen studies met inclusion criteria among 550 potentially relevant studies. The pooled analysis of 38,598 older adults showed that vitamin D supplementation decreased the odds of having at least one fall by 1% (odds ratio [OR]=1.01; 95% confidence interval [CI], 0.92–1.11; p=0.86); however, the difference was not statistically significant. Of eight studies with 19,946 older adults, the pooled analysis showed a 12% (OR=1.12; 95% CI, 0.97–1.29; p=0.11) decrease in the odds of having at least one fracture among older adults; however, the difference was also not statistically significant. Pooled subgroup analysis showed that neither low (<2,000 IU/day) nor high (≥2,000 and <4,000 IU/day) doses of vitamin D supplementation had any significant effect on the incidence of falls and fractures.
Falls is the most frequent accident type and the main reason for injury-related hospitalizations in adults aged 65 years and older.1) Three million older adults receive emergency room care each year for fall-related injuries.2) Injuries caused by falls are associated with increased mortality.3) The World Health Organization reports that falls are the second largest cause of unintentional injury death globally, leading to >684,000 deaths annually.4) Although all people are at risk of falls, age, sex, unsafe environments, socioeconomic factors, medication, and health of an individual can impact the risk of falling.5) Recent epidemiological studies support a relationship between vitamin D and increased muscle function related to the prevention of injurious falls.6,7)
Ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) are the two primary forms of vitamin D, a fat-soluble nutrient that is present in plants as vitamin D2 and as vitamin D3 in humans and animals. Vitamin D is primarily produced by the skin upon exposure to ultraviolet (UV) rays from the sun, supplements, and food. Vitamin D increases calcium and phosphate levels, which are crucial for bone formation and muscle contractions, immune system function, and glucose metabolism.8,9) Vitamin D deficiency is a cause for concern, especially in older adults, due to its association with poor physical performance, increased risk of falling, and osteoporosis-related fractures.10,11) Moreover, vitamin D may play a role in preserving or enhancing muscle strength, function, physical performance, and balance in older adults, as well as in lowering hip and non-vertebral fractures.12-14) By enhancing intestinal calcium absorption, vitamin D may enhance bone health and improve bone mineralization.15)
One study reported that 24 weeks of vitamin D supplementation reduced the incidence of falls in older adult populations.16) However, several systematic reviews concluded that vitamin D and calcium supplementation did not improve fracture or fall risks or bone mineral density.17,18) Recent studies showed no significant influence of vitamin D on the risk of injuries such as fractures compared to placebo19) and on falls among older adults.20) Therefore, the effects of vitamin D on fall prevention remain inconclusive. Therefore, the present comprehensive review of the literature and meta-analysis of randomized controlled trials (RCTs) aimed to establish the overall effectiveness of vitamin D in reducing falls in older persons.
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022383154) and was conducted in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.21) We conducted a review based on literature identified in the Cochrane Library, Embase, PubMed, and CINAHL databases. A manual search was conducted by looking at the reference lists of published studies and Google searches. The search was performed using the combinations of keywords and Medical Subject Headings (MeSH) terms of “accidental falls” OR “falling” OR “falls” OR “slip and fall” AND “vitamin deficiency” OR “vitamin D” OR “calciferol” AND “aged” OR “elderly” OR “frail elderly” OR “older people” and without date of publication and language restrictions (Table 1). The last update of the search was conducted on December 12, 2022.
EndNote version 20.1 was used to thoroughly screen each article in the databases. Three independent reviewers systematically screened the articles to identify relevant studies. The study inclusion criteria based on the Population, Intervention, Comparison, Outcome and Study (PICOS) criteria included: (1) older community-dwelling or institutionalized adults (≥60 years of age); (2) no use of vitamin D and calcium supplements at screening; (3) a control group taking a placebo or other complement such as calcium at the same dosage in all groups; (4) primary outcome of the assessment of the number of people with at least one fall and the secondary outcomes of the number of people with at least one fracture; and (5) RCT study design.
Articles meeting any of the following exclusion criteria were excluded: (1) not pertaining to the study topic; (2) irrelevant population, (3) non-research articles or inappropriate study design (such as a case-cross over, cross-sectional study, or case-control study); (4) study protocol; (5) an inappropriate outcome regarding the number of falls among the participants; (6) reporting from the same data set as other RCTs; (7) insufficient data after email requests to eligible authors for missing information; and (8) lack of full text.
The extracted data included study identity, study characteristics (author, year of publication, title, study design, and country), participant characteristics (study setting, sample size, mean age, and sex), intervention characteristics (frequency, duration, and dose), and outcomes (number of people who had at least one fall and/or number of people who had at least one fracture).
The risks of bias in the eligible RCTs were independently assessed by three reviewers following the approach in the Cochrane Handbook version 2 for a systematic review of interventions. Version 2 of the Cochrane risk of bias tool for randomized trials (RoB 2) is the instrument recommended for assessing the risk of bias in randomized trials. With a specific focus on various facets of trial design, conduct, and reporting, the RoB 2 is organized into a predetermined set of bias domains. A set of inquiries (referred to as "signaling questions") within each domain seeks to elicit details about trial characteristics that are important to the risk of bias. Based on the responses to the signaling questions, an algorithm generates a proposed conclusion regarding the probability of bias originating from each domain.
We assessed the accumulated evidence using RevMan version 5.4.1 (Copenhagen, Nordic Cochrane Center; The Cochrane Collaboration, 2020). The number of participants who fell at least once and sustained a fracture was statistically estimated in a standard event and total format with 95% confidence intervals (CIs).
We calculated the Q and I2 statistics to evaluate the heterogeneity of treatment effects across studies. I2 indicates the consistency of a study’s findings. The result is interpreted as the fraction of the overall variation across studies, which may be attributed to heterogeneity rather than random variance.22) We applied a scale of low, moderate, and high heterogeneity, with upper limits of 25%, 50%, and 75% for I2, respectively.23) Models for random effects were also computed. The analysis applied a random-effects model to account for differences between the included studies.24)
We conducted a subgroup analysis among the included studies.22) We conducted subgroup analyses for several potential moderator variables, including vitamin D type (vitamin D2 or vitamin D3) and doses (vitamin D2 or D3; low dose <2,000 IU/day, high dose ≥2,000 and <4,000 IU/day).25) We transformed the doses of vitamin D from either monthly or annual to daily intake.
Searches of the Embase, Cochrane, PubMed, and CINAHL electronic databases using the PRISMA flowchart identified 550 studies. Eighty studies were identified as duplicate records and were removed from the screening of the titles and abstracts. An additional 40 studies were excluded because of irrelevant topics, 69 studies had irrelevant populations, 36 studies were either reviews or meta-analyses, three studies were non-research articles, two studies were protocols, and 298 studies had irrelevant study designs. Therefore, the full texts of 22 studies were selected. Among these, one study was excluded because it was a duplicate publication, and 12 other studies were excluded because they did not measure the outcome of interest in this study. The manual, Google Scholar, and citation searches yielded 12 studies. Four of these studies were excluded because the populations were irrelevant. Finally, 17 studies presented sufficient information for data extraction and were eligible for enrollment in our study (Fig. 1).
Among 47,206 older adults enrolled in this study among the 17 included studies, 19,313 received vitamin D interventions, and 19,298 were assigned to placebo control groups. The mean ages ranged from 61.0 to 85.4 years. Approximately 25,361 patients (53.8%) were women. The included studies were conducted in Switzerland (17.6%), the United Kingdom (35.2%), Australia (23.6%), the United States (17.6%), and Germany (6.0%). The populations of the studies were all older adults and were heterogeneous. Most of the study participants were community-dwelling. The number of participants in the study ranged from 68 to 21,315, and the follow-up duration varied from 4 to 62 months. The vitamin D regimens in the intervention groups varied between studies in terms of the dose and method of administration. Most of the studies compared the effects of vitamin D3 supplementation among the intervention groups compared to the control group mostly administered placebos. Fifteen of the 17 studies indicated that administration occurred via the oral route (Table 2).16,26-41)
We assessed the risk of bias for all 17 included studies using the Cochrane Library’s RoB 2 tool. Among the assessed domains, all included studies had low risks of bias due to missing outcome data, measurement of outcomes, and selection of reported results. Regarding the risk of bias due to the randomization process, four studies16,30,36,39) were of some concern, while the other studies were of low risk. Regarding the risk of bias due to deviations from the intended interventions, only one study39) was of concern, whereas the others were of low risk. The overall risk of bias showed 13 with low risk and four studies with “some concern” (Table 2). The risks of bias according to the five domains are presented in Supplemental Table S1..
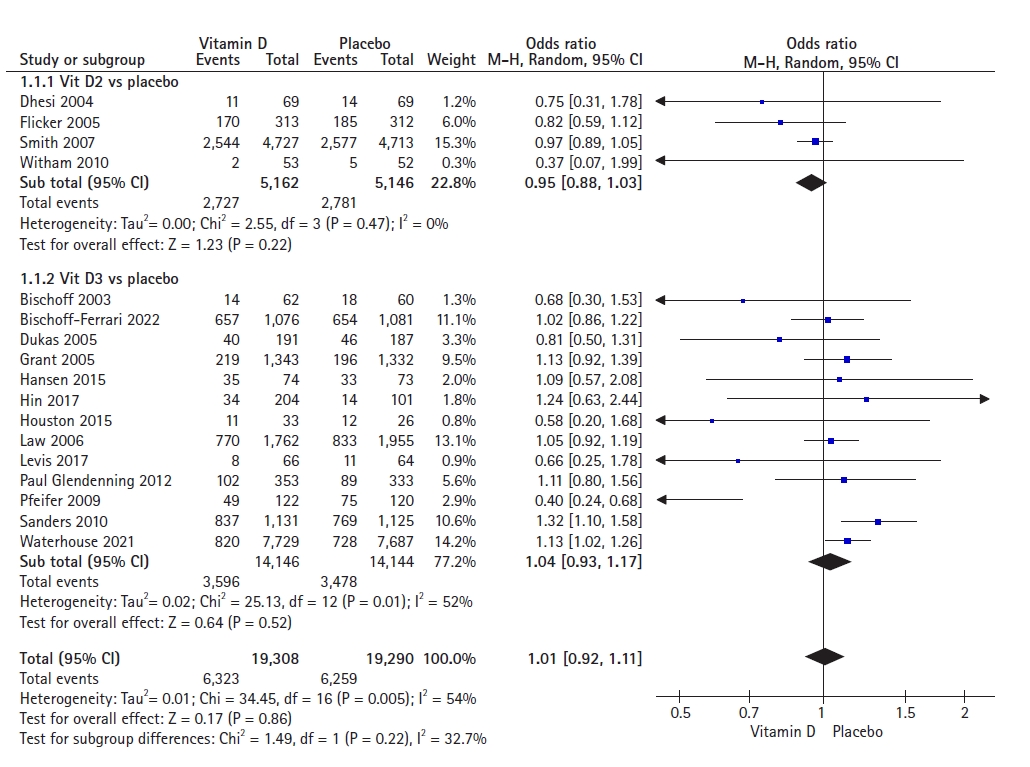
The forest plot of the effects of vitamin D (D2 and D3) supplementation on fall incidence included 17 studies with a total population of 38,598 individuals. The pooled result showed an odds ratio (OR) of 1.01 (95% CI, 0.92–1.11), indicating that vitamin D supplementation decreased the odds of having at least one fall by only 1% compared to placebo among older adults. The incidence of falls did not differ significantly between the vitamin D and placebo groups (p=0.86). We observed moderate statistical heterogeneity (I2=54%) (Fig. 2).
A subgroup analysis including four studies involving 10,308 participants to assess the effects of vitamin D2 supplementation showed no statistically significant reduction in fall incidence between the two groups (OR=0.95; 95% CI, 0.88–1.03; p=0.22). We observed no heterogeneity among the studies (I2=0%).
A subgroup analysis of 13 studies involving 28,290 participants to assess the effects of vitamin D3 supplementation showed no statistically significant reduction in fall incidence between the two groups (OR=1.04; 95% CI, 0.93–1.17; p=0.52). We observed moderate heterogeneity among the studies (I2=52%).
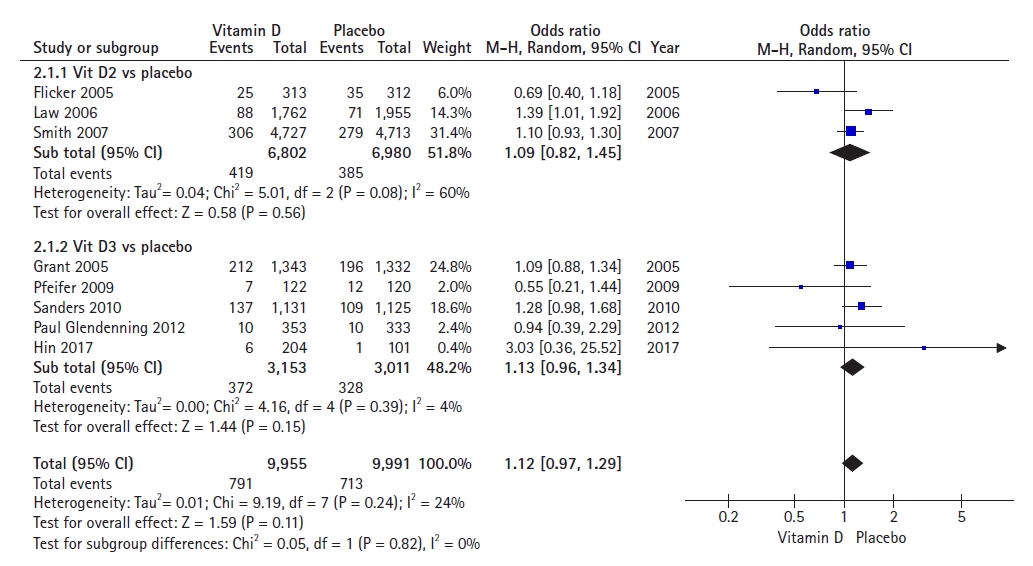
The pooled results of the forest plot of eight studies on vitamin D (D2 and D3) (Fig. 3) and fracture incidence among older adults, with a total population of 19,946 showed an OR of 1.12 (95% CI, 0.97–1.29), indicating that vitamin D supplementation decreased the odds of having at least one fracture by 12% compared to placebo among older adults. The incidence of fractures did not differ significantly between the vitamin D and placebo groups (p=0.11). We observed low statistical heterogeneity in this meta-analysis (I2=24%).
A subgroup analysis of three studies on vitamin D2 supplementation revealed no statistically significant reduction in fracture incidences between the two groups (OR=1.09; 95% CI, 0.82–1.45; p=0.56). The analysis included a total of 13,782 participants. High heterogeneity was observed among these studies (I2=60%).
The results of the subgroup analysis of five studies that administered vitamin D3 supplementation showed no statistically significant reduction in fracture incidences between the two groups (OR=1.13; 95% CI, 0.96–1.34; p=0.15). The analysis included a total of 6,164 participants included in the analysis. Low heterogeneity was observed among studies (I2=4%).
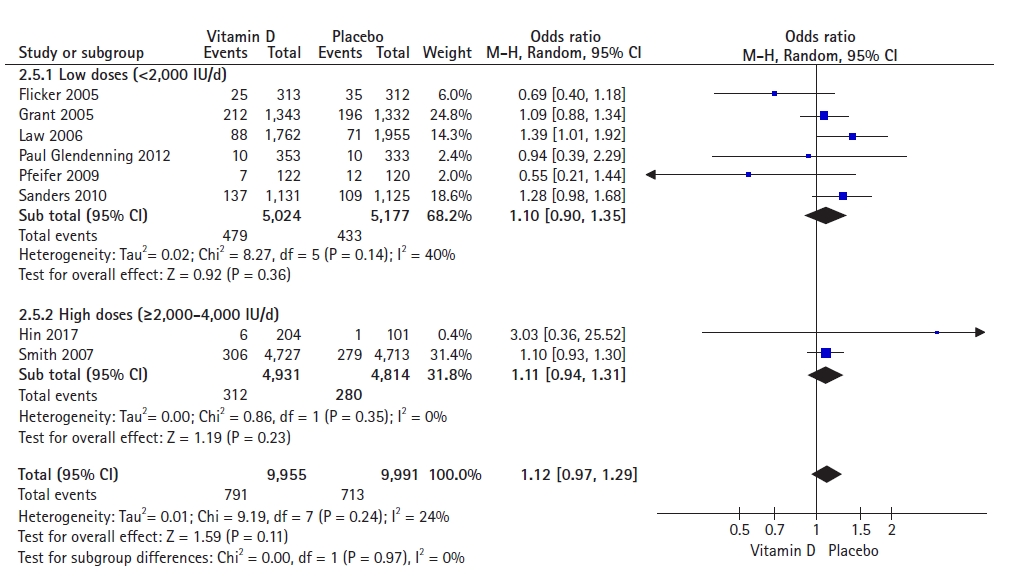
A pooled analysis of the effects of low doses (<2,000 IU/day) including nine studies with 10,806 participants and high doses (≥2,000 and <4,000 IU/day) including eight studies with 27,792 participants on fall incidence showed an OR of 0.95 (95% CI, 0.78–1.14; p=0.56) and OR of 1.03 (95% CI, 0.95–1.11; p=0.50). These results indicated no significant difference between the two groups. We observed high heterogeneity for low doses (I2=69%) and low heterogeneity for high doses (I2=16%) (Fig. 4).
A pooled analysis of low doses (<2,000 IU/day) including six studies with 10,201 participants and high doses (≥2,000 and <4,000 IU/day) including two studies on fracture incidence that enrolled 9,745 participants, which reported OR values of 1.10 (95% CI, 0.90–1.35; p=0.36) and OR of 1.11 (95% CI, 0.94–1.31; p=0.23). These results indicated a lack of significant difference between the two groups. Low heterogeneity was observed in both subgroups (I2<50%) (Fig. 5).
Our meta-analysis included 17 studies with 47,206 older adults who received vitamin D supplementation or a placebo for 5 months to 5 years. The total population of all included studies and the total population of the intervention and placebo groups differed because the participants were not followed up to the end of the study,26) they were divided into more than just vitamin D intervention or placebo groups in some studies,26-28) or they were moved to long-term care or died during the study period.29)
We observed no significant difference in the likelihood of falls among people who took vitamin D supplements versus the placebo group for either vitamin D2 or vitamin D3 products. The results of our meta-analysis support those of a previous study showing that vitamin D treatment alone did not result in lower falls in older persons with D3 levels >50 nmol/L.20) However, we did not analyze the effect of vitamin D supplements based on D3 concentrations measured in the participants' bodies. More studies, like RCTs or meta-analyses, etc. are needed to analyze the effects of vitamin D supplements on the concentrations of D3 in participants' bodies measured before they take these supplements. Previous meta-analyses have also demonstrated the effectiveness of vitamin D in reducing the incidence of falls among older adults.41-43)
Our results differed due to the contradictory results of the included randomized studies. The pooled results of the analysis of four RCTs that used vitamin D2 as an intervention showed that vitamin D2 reduced the number of older adults falling in all studies, however, the difference was not statistically significant.30-33) In contrast, among 13 studies that used vitamin D3 as an intervention, five RCTs showed that vitamin D3 reduced the risk of falls in older adults,16,29,34-36) while the remaining eight revealed the opposite, in which that vitamin D3 supplementation increased the number of people falling.26-28,37-41) Sanders et al.41) and Waterhouse et al.27) showed that vitamin D3 significantly increased the number of older adults falling compared to placebo. This raises the possibility of adverse outcomes for high doses of vitamin D3. In 2010, Sanders et al.41) administered an annual dose of 500,000 IU of cholecalciferol to the intervention group. Similarly, in 2012, Yang45) recommended monthly vitamin D doses of 24,000 IU as a daily supplement. Finally, a recent study by Waterhouse et al.27) administered 60,000 IU of cholecalciferol monthly.
A major concern in studies on falls in older adults is the occurrence of fractures following falls. Similarly, analyses of fracture outcomes revealed no statistically significant difference in fracture risk between older adults who received vitamin D2, vitamin D3, or a placebo. Among the RCTs that administered vitamin D2 as an intervention, two showed that vitamin D2 increased the number of older adults with fractures32,39); the other showed that vitamin D2 reduced the number of fractures.31) However, these results were not statistically significant. The same was true for vitamin D3, with two RCTs showing that vitamin D3 reduced the risk of fractures36,40) and another three showing an increased number of fractures in the vitamin D3 group.28,38,41) The results of our meta-analysis were similar to those of a previous study reporting that vitamin D3 supplementation did not significantly reduce fracture risk compared to placebo among older adults.19) However, another meta-analysis on the combination of vitamin D3 and calcium showed the opposite results. Using a daily oral supplement, the results showed that supplementation with 800 IU of vitamin D3 combined with 1,200 mg of calcium reduced the risks of hip and non-vertebral fractures.12) These findings raise the possibility of an effective combination of calcium and vitamin D to reduce the fracture risk in older adults. Future RCTs are needed to further investigate the results of this combination with different doses and timings of vitamin D administration, and with other uses such as intramuscular injection.
After converting the vitamin D dose in the intervention groups of all RCTs to daily dosing, the results of the meta-analyses showed no statistically significant differences in the number of older adults with falls and fractures between the vitamin D intervention and placebo groups. Most RCTs showed no statistically significant difference in falls; however, Pfeifer et al.36) demonstrated the protective effect of vitamin D, specifically vitamin D3. The authors observed a statistically significant reduction in the number of older adults with falls compared with the placebo. In contrast, Sanders et al.41) showed a higher number of older adults with falls in the vitamin D3 group compared to in the placebo group; however, the difference was not significantly significant. However, the final pooled result of all RCTs revealed no significant difference.
The major strength of our study is that it included 17 RCTs with many total participants, including 47,206 older adults from different countries worldwide. In addition to the pooled results of the total included studies, sub-analyses of vitamins D2 and D3 were also performed to determine the results for different forms of vitamin D. However, our study also has several limitations. Most of the participants were from Western countries, except for one study that included Caucasian, Afro-Caribbean, and Middle Eastern participants30); moreover, none of the included studies was conducted in Asian regions. Another limitation was that our meta-analysis considered only English-language publications. All of the above limitations underscore the need for future studies to identify relevant studies in populations in all regions, with no restriction on languages from databases to obtain more data to analyze the effect and safety of vitamin D supplementation. Additional meta-analyses of other subgroups such as oral administration groups, injection-using groups, and short- and long-term use are needed to support shared decision-making for a nutritionist to help patients make good choices regarding vitamin D supplementation.
In conclusion, the effects of vitamin D on the promotion of calcium and phosphate concentrations for bone growth and muscle activity are well-known. However, evidence for the effect of vitamin D in reducing falls remains inconsistent. The results of our study showed that, compared to a placebo, vitamin D supplementation did not significantly reduce the incidence of falls or fractures in older adults.
ACKNOWLEDGMENTS
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4235/agmr.23.0047.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of included studies.
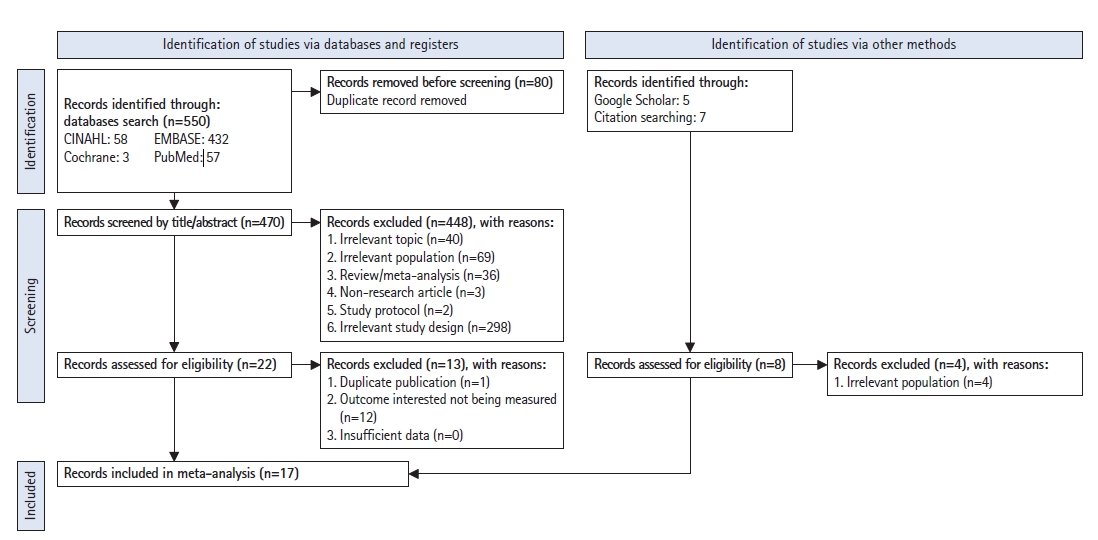
Table 1.
Search string databases
Table 2.
Characteristics of randomized controlled trials included in the meta-analysis (n=17)
| Study | Year | Country | Study setting | Populations | Duration (mo) |
Group type |
Study quality (RoB Cochrane 2.0) | ||
|---|---|---|---|---|---|---|---|---|---|
| Experiment | Control | ||||||||
| 1 | Bischoff et al.34) | 2003 | Switzerland | Long-stay geriatric care | Mean age: 85.3 y | 4 | Vitamin D3+calcium | Calcium | Low |
| Sample size: 122 | Dose: 800 IU (vitamin D) daily | ||||||||
| Sex: male 0 (0%), female 122 (100%) | Route: per oral | ||||||||
| 2 | Bischoff-Ferrari et al.37) | 2022 | Switzerland, Germany, Austria, France, and Portugal | Community | Mean age: 74.9 y | 36 | Vitamin D3 | Placebo | Low |
| Sample size: 2,157 | Dose: 2,000 IU daily | ||||||||
| Sex: male 826 (38.3%), female 1,331 (61.7%) | Route: per oral | ||||||||
| 3 | Dhesi et al.30) | 2004 | UK | Community | Mean age: 76.8 y | 6 | Vitamin D2 | Placebo | Some concerns |
| Sample size: 138 | Dose: 600,000 IU per 6 month (~3,000 IU daily) | ||||||||
| Sex: male 30 (21.7%), female 108 (78.3) | Route: intramuscular | ||||||||
| 4 | Dukas et al.16) | 2005 | Switzerland | Community | Mean age: 75.0 y | 9 | Vitamin D3 | Placebo | Some concerns |
| Sample size: 378 | Dose: 1 µg alphaD3 capsules (40 IU daily) | ||||||||
| Sex: male 163 (43.1%), female 215 (56.9%) | Route: per oral | ||||||||
| 5 | Flicker et al.31) | 2005 | Australia | Nursing home | Mean age: 85.4 y | 24 | Vitamin D2 | Placebo | Low |
| Sample size: 625 | Dose: 10,000 IU given once weekly (~1,000 IU daily) | ||||||||
| Sex: male 32 (5.1%), female: 593 (94.9%) | Route: per oral | ||||||||
| 6 | Glendenning et al.40) | 2012 | Australia | Community | Mean age: 76.7 y | 9 | Vitamin D3 | Placebo | Low |
| Sample size: 686 | Dose: 150,000 IU every 3 months (~1,600 IU daily) | ||||||||
| Sex: male 0 (0%), female 686 (100%) | Route: per oral | ||||||||
| 7 | Grant et al.28) | 2005 | UK | Hospital | Mean age: 77.0 y | 62 | Vitamin D3 | Placebo (calcium) | Low |
| Sample size: 5,292 | Dose: 800 IU daily | ||||||||
| Sex: male 811 (15.3%), female 4,481 (84.7%) | Route: per oral | ||||||||
| 8 | Hansen et al.26) | 2015 | USA | Institutionalized | Mean age: 61.0 y | 12 | Vitamin D3 | Placebo | Low |
| Sample size: 230 | Dose: 50,000 IU twice monthly (~3,000 IU daily) | ||||||||
| Sex: male 0 (0%), female: 230 (100%) | Route: per oral | ||||||||
| 9 | Hin et al.38) | 2017 | UK | Community | Mean age: 72.0 y | 12 | Vitamin D3 | Placebo | Low |
| Sample size: 305 | Dose: 2,000 IU daily and 4,000 IU daily | ||||||||
| Sex: male 155 (50.8%), female 150 (49.2%) | Route: per oral | ||||||||
| 10 | Houston et al.29) | 2015 | USA | Community | Mean age: 77.9 y | 5 | Vitamin D3 | Placebo (vitamin E) | Low |
| Sample size: 68 | Dose: 100,000 IU per month (~3,000 IU daily) | ||||||||
| Sex: male 19 (27.9%), female 49 (72.1%) | Route: per oral | ||||||||
| 11 | Law et al.39) | 2006 | UK | Nursing home | Mean age: 85.0 y | 10 | Vitamin D3 | Control | Some concerns |
| Sample size: 3,717 | Dose: 1,100 IU daily | ||||||||
| Sex: male 892 (24.0%), female 2,825 (76.0%) | Route: per oral | ||||||||
| 12 | Levis et al.35) | 2017 | USA | Institutionalized | Mean age: 72.4 y | 9 | Vitamin D3 | Placebo | Low |
| Sample size: 130 | Dose: 4,000 IU daily | ||||||||
| Sex: male 130 (100%), female 0 (0%) | Route: per oral | ||||||||
| 13 | Pfeifer et al.36) | 2009 | Germany | Community | Mean age: 77.0 y | 12 | Vitamin D3+calcium | Placebo (calcium) | Some concerns |
| Sample size: 242 | Dose: 800 IU daily (vitamin D3) | ||||||||
| Sex: male 123 (50.8%), female 119 (49.2%) | Route: per oral | ||||||||
| 14 | Sanders et al.41) | 2010 | Australia | Community | Mean age: 76.3 y | 60 | Vitamin D3 | Placebo | Low |
| Sample size: 2,256 | Dose: 500,000 IU annually (~1,300 IU daily) | ||||||||
| Sex: male 0 (0%), female 2,256 (100%) | Route: per oral | ||||||||
| 15 | Smith et al.32) | 2007 | UK | Community | Mean age: 79.4 y | 36 | Vitamin D2 | Placebo | Low |
| Sample size: 9,440 | Dose: 300,000 IU annually every autumn (~3,000 IU daily) | ||||||||
| Sex: male 4,354 (46.1%), female 5,086 (53.9%) | Route: intramuscular | ||||||||
| 16 | Waterhouse et al.27) | 2021 | Australia | Community | Mean age: 69.3 y | 60 | Vitamin D3 | Placebo | Low |
| Sample size: 21,315 | Dose: 60,000 IU monthly (~2,000 IU daily) | ||||||||
| Sex: male 14,241 (66.8%), female 7,074 (33.2%) | Route: per oral | ||||||||
| 17 | Witham et al.33) | 2010 | UK | Community | Mean age: 79.7 y | 5 | Vitamin D2 | Placebo | Low |
| Sample size: 105 | Dose: 100,000 IU per 4 months (~600 IU daily) | ||||||||
| Sex: male 69 (65.7%), female 36 (34.3%) | Route: per oral | ||||||||
REFERENCES
1. Gale CR, Cooper C, Aihie Sayer A. Prevalence and risk factors for falls in older men and women: The English Longitudinal Study of Ageing. Age Ageing 2016;45:789–94.



2. Moreland B, Lee R. Emergency department visits and hospitalizations for selected nonfatal injuries among adults aged ≥65 years: United States, 2018. MMWR Morb Mortal Wkly Rep 2021;70:661–6.



3. Appeadu MK, Bordoni B. Falls and fall prevention in the elderly. Tampa, FL: StatPearls Publishing; 2022.
4. World Health Organization. Falls [Internet]. Geneva, Switzerland: World Health Organization; 2021 [cited 2023 Sep 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/falls.
5. While AE. Falls and older people: understanding why people fall. Br J Community Nurs 2020;25:173–7.


6. Sim M, Zhu K, Lewis JR, Hodgson JM, Prince RL. Association between vitamin D status and long-term falls-related hospitalization risk in older women. J Am Geriatr Soc 2021;69:3114–23.



7. Neale RE, Wilson LF, Black LJ, Waterhouse M, Lucas RM, Gordon LG. Hospitalisations for falls and hip fractures attributable to vitamin D deficiency in older Australians. Br J Nutr 2021;126:1682–6.


8. Sintov AC, Yarmolinsky L, Dahan A, Ben-Shabat S. Pharmacological effects of vitamin D and its analogs: recent developments. Drug Discov Today 2014;19:1769–74.


9. Charoenngam N, Shirvani A, Holick MF. Vitamin D for skeletal and non-skeletal health: what we should know. J Clin Orthop Trauma 2019;10:1082–93.



10. Uusi-Rasi K, Patil R, Karinkanta S, Tokola K, Kannus P, Lamberg-Allardt C, et al. Serum 25-hydroxyvitamin D levels and incident falls in older women. Osteoporos Int 2019;30:93–101.



11. Moon H, Ko HJ, Kim AS. The relationship between serum 25-hydroxyvitamin D levels and physical performance in community-dwelling older adults. Ann Geriatr Med Res 2019;23:9–15.



12. Manoj P, Derwin R, George S. What is the impact of daily oral supplementation of vitamin D3 (cholecalciferol) plus calcium on the incidence of hip fracture in older people?: a systematic review and meta-analysis. Int J Older People Nurs 2023;18:e12492.


13. Bischoff-Ferrari HA. Relevance of vitamin D in muscle health. Rev Endocr Metab Disord 2012;13:71–7.



14. Granic A, Hill TR, Davies K, Jagger C, Adamson A, Siervo M, et al. Vitamin D status, muscle strength and physical performance decline in very old adults: a prospective study. Nutrients 2017;9:379.



15. Fleet JC, Schoch RD. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci 2010;47:181–95.



16. Dukas L, Schacht E, Mazor Z, Stahelin HB. Treatment with alfacalcidol in elderly people significantly decreases the high risk of falls associated with a low creatinine clearance of <65 ml/min. Osteoporos Int 2005;16:198–203.



17. Gallagher JC. Vitamin D and bone density, fractures, and falls: the end of the story? Lancet Diabetes Endocrinol 2018;6:834–5.


18. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol 2018;6:847–58.


19. LeBoff MS, Chou SH, Ratliff KA, Cook NR, Khurana B, Kim E, et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med 2022;387:299–309.



20. Ling Y, Xu F, Xia X, Dai D, Xiong A, Sun R, et al. Vitamin D supplementation reduces the risk of fall in the vitamin D deficient elderly: an updated meta-analysis. Clin Nutr 2021;40:5531–7.


21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:89.


22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.



23. Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect 2014;20:123–9.


24. Serghiou S, Goodman SN. Random-effects meta-analysis: summarizing evidence with caveats. JAMA 2019;321:301–2.


25. Bleizgys A. Vitamin D dosing: basic principles and a brief algorithm (2021 update). Nutrients 2021;13:4415.



26. Hansen KE, Johnson RE, Chambers KR, Johnson MG, Lemon CC, Vo TN, et al. Treatment of vitamin D insufficiency in postmenopausal women: a randomized clinical trial. JAMA Intern Med 2015;175:1612–21.



27. Waterhouse M, Sanguineti E, Baxter C, Duarte Romero B, McLeod DS, English DR, et al. Vitamin D supplementation and risk of falling: outcomes from the randomized, placebo-controlled D-Health Trial. J Cachexia Sarcopenia Muscle 2021;12:1428–39.




28. Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 2005;365:1621–8.


29. Houston DK, Tooze JA, Demons JL, Davis BL, Shertzer-Skinner R, Kearsley LB, et al. Delivery of a vitamin D intervention in homebound older adults using a meals-on-wheels program: a pilot study. J Am Geriatr Soc 2015;63:1861–7.




30. Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing 2004;33:589–95.


31. Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA, et al. Should older people in residential care receive vitamin D to prevent falls?: results of a randomized trial. J Am Geriatr Soc 2005;53:1881–8.


32. Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women: a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford) 2007;46:1852–7.


33. Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail 2010;3:195–201.


34. Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res 2003;18:343–51.


35. Levis S, Gomez-Marin O. Vitamin D and physical function in sedentary older men. J Am Geriatr Soc 2017;65:323–31.



36. Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int 2009;20:315–22.



37. Bischoff-Ferrari HA, Freystatter G, Vellas B, Dawson-Hughes B, Kressig RW, Kanis JA, et al. Effects of vitamin D, omega-3 fatty acids, and a simple home strength exercise program on fall prevention: the DO-HEALTH randomized clinical trial. Am J Clin Nutr 2022;115:1311–21.



38. Hin H, Tomson J, Newman C, Kurien R, Lay M, Cox J, et al. Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos Int 2017;28:841–51.




39. Law M, Withers H, Morris J, Anderson F. Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in elderly people in residential accommodation. Age Ageing 2006;35:482–6.


40. Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res 2012;27:170–6.


41. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303:1815–22.


42. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomized controlled trials. BMJ 2009;339:b3692.



43. Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of vitamin D on falls: a meta-analysis. JAMA 2004;291:1999–2006.