Long-Term Risk of Reduced Cognitive Performance and Associated Factors in Discharged Older Adults with COVID-19: A Longitudinal Prospective Study
Article information
Abstract
Background
Increasing numbers of reports have suggested a deterioration in cognitive performance after recovery from coronavirus disease 2019 (COVID-19), however insufficient information is available regarding long-term brain health and risk factors related to reduced cognitive performance in advanced age. We investigated the prevalence of reduced cognitive performance and its associated factors among older adults after COVID-19.
Methods
This prospective observational study enrolled older individuals (aged ≥65 years) hospitalized for COVID-19. Discharged patients were contacted after an average of 15 months and a brief battery was administered during telephone interviews to assess their mental status.
Results
Among the 174 patients, 77 (44.3%) showed reduced cognitive performance at follow-up. Multivariate analysis revealed that female sex, education level, and increased Deyo/Charlson Comorbidity Index score, which is an objective indicator of chronic disease burden, were independent risk factors for long-term cognitive performance. Depression and anxiety symptoms, assessed using the Patient Health Questionnaire-2 and Generalized Anxiety Disorder 2-item questionnaire at the end of the study, were not associated with reduced cognitive performance.
Conclusion
Our findings provide key insights into discharged older adults with COVID-19 at risk of long-term cognitive impairment, and help to ascertain the factors associated with this problem.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) has adverse effects on the respiratory system in the acute phase; however, increasing evidence suggests that the disease also affects the musculoskeletal, gastrointestinal, neurological, and cardiovascular systems.1,2) Recently, the long-term symptoms of this disease, termed "post-COVID" syndrome by the World Health Organization, have been characterized as a series of unexplained findings, usually appearing 3 months after the infection onset and lasting for >2 months thereafter.3,4) Moreover, recent reports have shown that the virus can cause neurological manifestations such as headache, dizziness, confusion, and cognitive dysfunction, although the reasons are not fully understood.1,3)
The prevalence of poor overall cognitive performance after 3–6 months following hospital discharge varies from 21% to 65%.5,6) Furthermore, heterogeneous results indicate reduced performance in cognitive functions such as memory, attention, processing speed, problem-solving, planning, reasoning, and visuospatial ability shortly after COVID-19 onset.7-9) The results of neuropsychiatric assessments performed 3–6 months after infection suggest that cognitive changes may develop during long-term follow-up.5-10) A longitudinal study of 21 patients in Italy reporting subjectively reduced cognitive performance showed that 52% of the patients had deficits in at least one cognitive domain at 6 months, a rate that gradually decreased after 18 months.11) Another recent study reported that the rate of cognitive performance decline accelerated four-fold in patients with reduced cognitive performance compared to the pre-pandemic period.12)
Findings on the factors associated with reduced cognitive performance following COVID-19 disease are conflicting. Neuroinflammation, hypoxia, and procoagulatory and prothrombotic states may be underlying conditions leading to reduced cognitive performance after COVID-19.13,14) Furthermore, some studies have highlighted post-intensive care syndrome, which may manifest as the deterioration of cognitive and physical functions in patients surviving intensive care owing to COVID-19.15-18) Furthermore, hospitalization, delirium, social isolation, depression, and anxiety owing to the loss of loved ones or fear of death may negatively affect cognitive performance.19,20) Several studies have reported no association between cognition and the length of hospital stay, oxygen demand, comorbidities, or inflammation.6) However, studies to date have been conducted in the general population and have not focused on older adults who are more vulnerable to permanent neuropsychiatric disorders and reduced cognitive performance.5-9)
Although evidence suggests that older individuals recovering from COVID-19 are at a greater risk of cognitive dysfunction, information is insufficient regarding the long-term cognitive status and risk factors in this population. Therefore, this prospective study sought to answer the following questions: What is the frequency of long-term decline in cognitive performance after hospitalization for COVID-19 in older adults without previously known cognitive impairment, and which factors affect cognitive performance impairment?
MATERIALS AND METHODS
Design and Participants
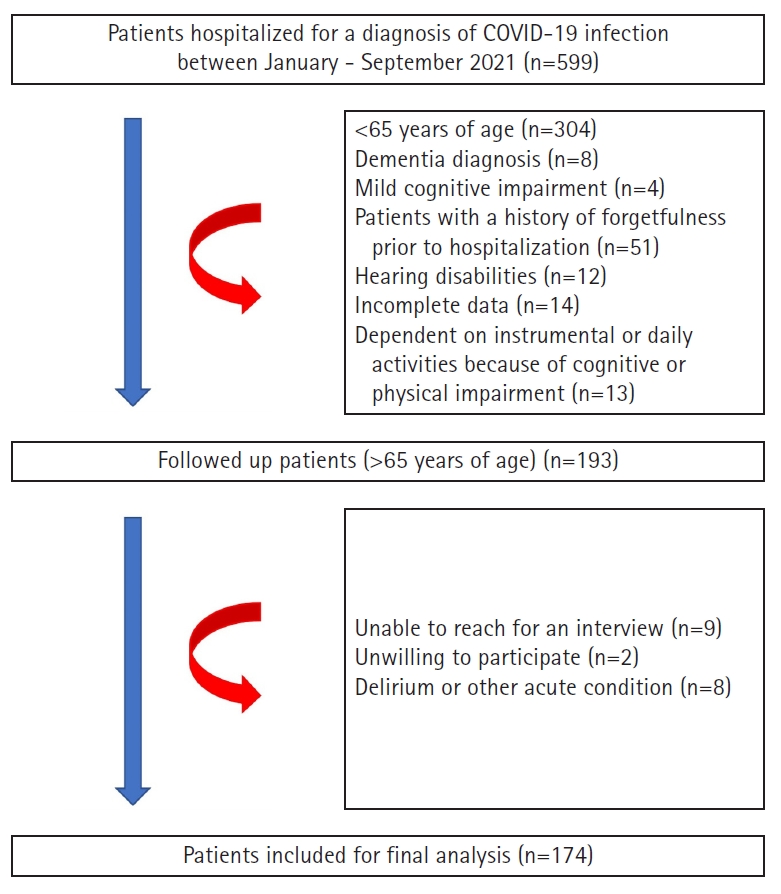
This prospective study recruited individuals aged ≥65 years hospitalized for COVID-19 at a tertiary hospital in Ankara, Türkiye, between January and September 2021. All patients tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by reverse transcription-polymerase chain reaction of a nasal swab specimen or had typical computed chest scan images (bilateral multifocal ground-glass opacities) compatible with COVID-19. Strict exclusion criteria were applied at the beginning of the study to include as many cognitively normal individuals as possible. Thus, we asked patients for their own verbal statements about their forgetfulness before the COVID-19 pandemic and confirmed these statements in the national database. All hospitals in our country use a common nationwide database of basic medical records (outpatient visits, surgeries, radiology, prescribed medications, and basic medical histories). Data obtained from patient interviews were compared and, when possible, confirmed using this national database. Patients who had previously complained of forgetfulness, presented to the outpatient clinic with these complaints, were diagnosed with mild cognitive impairment (MCI) or dementia, had been prescribed medication for dementia for any reason, experienced hearing or visual impairment, had incomplete data, had a terminal illness, or were bedridden were excluded from the study. Patients who were dependent on instrumental or daily activities because of cognitive or physical impairment in the pre-COVID-19 period were also excluded because MCI could not be ruled out (Fig. 1).
Patients who recovered and were discharged from the hospital were contacted between September 2021 and October 2022. Additional exclusion criteria were unavailability of telephone contact, lack of consent to participate in the study, presence of delirium, or medical instability owing to any illness such as acute infection or acute exacerbation of a chronic condition. The local ethics committee approved the study (No. 2020-305), which was conducted in accordance with the guidelines of the Declaration of Helsinki. All patients included in the study gave their written and verbal consent to participate in the study. Also, this study complied the ethical guidelines for authorship and publishing in the Annals of Geriatric Medicine and Research.21)
A single trained interviewer conducted a telephone-based visit for 30 minutes at the end of the follow-up period and obtained verbal informed consent from all included participants. The interviews began with an assessment of delirium and medical conditions. Subsequently, a brief battery was used to assess mental status, including the Patient Health Questionnaire-2 (PHQ-2), the Generalized Anxiety Disorder 2-item questionnaire (GAD-2), and the Telephone Cognitive Screening Scale Türkiye (T-CogS-TR). After the interview, cognitive performance was evaluated and recorded as present or not (categorically). Individuals with poor cognitive performance were verbally advised to visit a nearby health institution for further examination.
To calculate the minimum sample size required, the incidence of dementia in the population ≥65 years of age was assumed to be 0.5%, whereas after COVID-19 was assumed to be 2.66%.22) Thus, a total of 161 cases was calculated to be sufficient for maximum type 1 and type 2 errors of 0.05 and 0.20, respectively.
Mental Status Assessment
Mental health was assessed using the T-CogS-TR, PHQ-2, and GAD-2. The T-CogS-TR is a 16-question screening instrument used to identify reduced cognitive performance. This test evaluates cognitive functions including orientation, registration, memory, attention, and language. The overall scores range between 0 and 26 points, with a cut-off score of ≤21 points indicating reduced cognitive performance (sensitivity 96.8%, specificity 90.2%).23) The PHQ-2 is a self-reported depression screening tool that detects and measures depressive symptoms within the past 2 weeks. Two items are rated on a three-point Likert scale, with a cut-off score of ≥3 indicating depression (range, 0–6).24) The GAD-2 is a self-reported instrument used to assess generalized anxiety disorder symptoms within the past 2 weeks. Two items are rated on a three-point Likert scale, with a cut-off score of ≥3 indicating anxiety (range, 0–6).25)
Reduced Cognitive Performance
The outcome variable was reduced cognitive performance at follow-up. The participants were stratified into two groups according to their T-CogS-TR scores: reduced cognitive performance (≤21 points) and cognitively healthy (≥22 points).23)
Covariates
The potential confounders included basic demographics (age, sex, marital status, and educational level), medical history (chronic diseases and currently used medications), laboratory parameters (neutrophils, lymphocytes, hemoglobin, C-reactive protein [CRP], D-dimer, glomerular filtration rate [GFR], and lactate dehydrogenase [LDH]), intensive care unit (ICU) admission, delirium, and length of hospitalization. All data were obtained from patient charts and electronic clinical records. An unfavorable clinical outcome was linked to baseline neutrophil count <4×109/L, lymphocyte count <1×109/L, neutrophil/lymphocyte ratio >8, CRP >30 mg/L, D-dimer >0.5 mg/L, and LDH >300 U/L.26,27) Anemia was defined as hemoglobin levels <12 g/L in women and <13 g/L in men.28) Decreased kidney function was described as a GFR <60 mL/min/1.73 m2.29)
The Deyo/Charlson Comorbidity Index (DCCI), which gives a weighted score for each of 17 comorbidities (acquired immunodeficiency syndrome [AIDS], any hematological malignancy, cerebrovascular disease, chronic pulmonary disease, congestive heart failure, dementia, diabetes with complications, diabetes without chronic complications, hemiplegia or paraplegia, metastatic solid tumor, mild liver disease, moderate/severe liver disease, myocardial infarction, peptic ulcer disease, peripheral vascular disease, renal disease, and rheumatoid disease) according to the risk of mortality within 1 year, was used to assess the comorbidity level.30) The concomitant use of five or more drugs was defined as polypharmacy.31) The anticholinergic burden of drugs was evaluated based on the Anticholinergic Cognitive Burden scale,32) with high exposure classified as a score ≥1.
Statistical Analyses
All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). The Kolmogorov–Smirnov test was used to determine the data distributions. The chi-squared test was used to compare categorical variables. Student t-test was used for normally distributed continuous variables, Student t-test was used, whereas the Mann–Whitney U test was used for non-normally distributed variables. The effect size in Student t-test was calculated as the difference between the group means divided by their pooled standard deviation, known as Cohen’s d.33) In non-parametric comparisons, effect size was calculated by dividing the absolute standardized test statistic "z" by the square root of the number of pairs.33) Phi (φ) was used to indicate the effect size measure in the chi-square test.33) A univariate Cox regression analysis was performed to examine the association between time and reduced cognitive performance after follow-up. Subsequently, a multivariate Cox regression model was constructed using clinically and statistically significant (p<0.1) variables (age, sex, marital status, educational level, DCCI, D-dimer level, and delirium history). The effects of depressive and anxiety symptoms on cognitive impairment at the end of the follow-up period were assessed using univariate binary logistic regression analysis, followed by multivariate adjustments for age, sex, and educational status. The statistical significance level was set at p<0.05.
RESULTS
Basic Characteristics
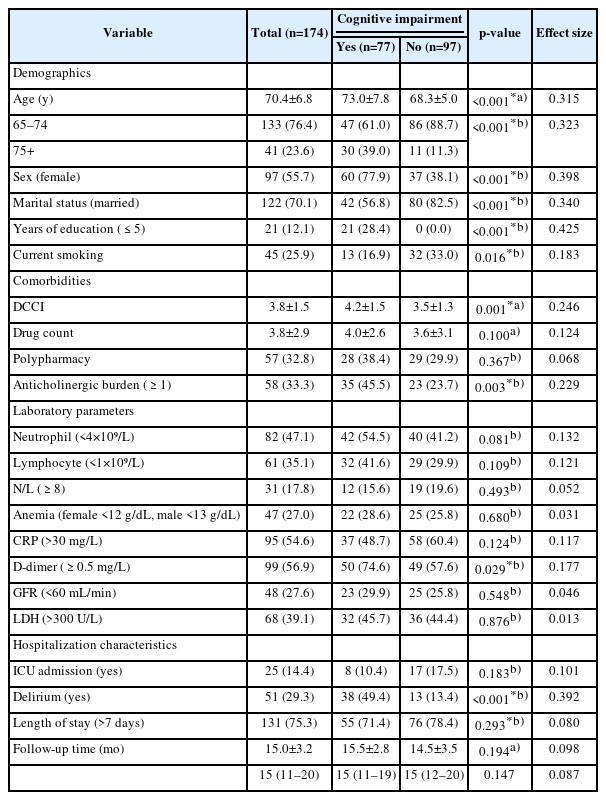
We enrolled 174 patients (mean age 70.4±6.8 years), with slight female predominance (55.7%). The mean follow-up period was 15.0±3.2 months (range, 11–18 months). At follow-up, 77 (44.3%) participants had cognitive impairment. Compared to patients without reduced cognitive performance, those who developed cognitive impairment were older (p<0.001, r=0.315); predominantly women (p<0.001, φ=0.398); unmarried (p<0.001, φ=0.340), had a lower educational level (≤5 years) (p<0.001, φ=0.425); and had greater current tobacco use (p=0.016, φ=0.183), DCCI score (p=0.001, r=0.246), anticholinergic burden ≥1 (p=0.003, φ=0.229), D-dimer level ≥0.5 (p=0.029, φ=0.177), and delirium history during hospitalization (p<0.001, φ=0.392). The number of medications, polypharmacy, other laboratory parameters, ICU admission, length of hospital stay, and follow-up period did not differ between the two groups. The characteristics of all participants are shown in Table 1.
Mental Health Status Assessment after Follow-Up
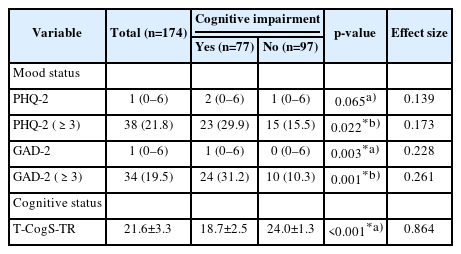
The mean score of all subjects on the T-CogS-TR questionnaire after follow-up was 21.6±3.3. The mean T-CogS-TR scores in patients with and without reduced cognitive performance were 18.7±2.5 and 24.0±1.3, respectively. The group with reduced cognitive performance had more clinically significant depression and anxiety symptoms (p=0.022, φ=0.173 and p=0.001, φ=0.261, respectively) (Table 2).
Associations between Cognitive Impairment and Clinical Variables
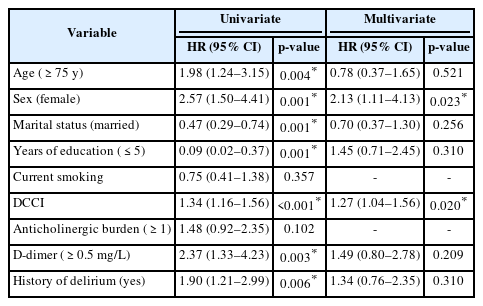
In the univariate Cox regression analyses, age (>75 years) (hazard ratio [HR]=1.98; 95% confidence interval [CI] 1.24–3.15; p=0.004), female sex (HR=2.57; 95% CI 1.50–4.41; p=0.001), 1-year decrease in education (HR=0.84; 95% CI 0.78–0.90; p<0.001), each point increase in DCCI score (HR=1.34; 95% CI 1.16–1.56; p<0.001), D-dimer (≥0.5 mg/L) (HR=2.37; 95% CI 1.33–4.23; p=0.003), and a history of delirium (HR=1.90; 95% CI 1.21–2.99; p=0.006) were associated with the presence of reduced cognitive performance. In the multivariable analysis, female sex (HR=1.27; 95% CI 1.04–1.56; p=0.020), 1-year decrease in education (HR=0.87; 95% CI 0.79–0.94; p=0.001) and each point increase in DCCI score (HR=1.34; 95% CI 1.16–1.56; p<0.001) were associated with reduced cognitive performance risk (Table 3, Fig. 2). The Hosmer–Lemeshow (H–L) test yielded a chi-square value of 11.913 and was insignificant (p=0.155), indicating a good fit of the model. The omnibus test confirmed that the model was highly significant (−2LL=119.161, χ2(2)=71.449, p<0.001).
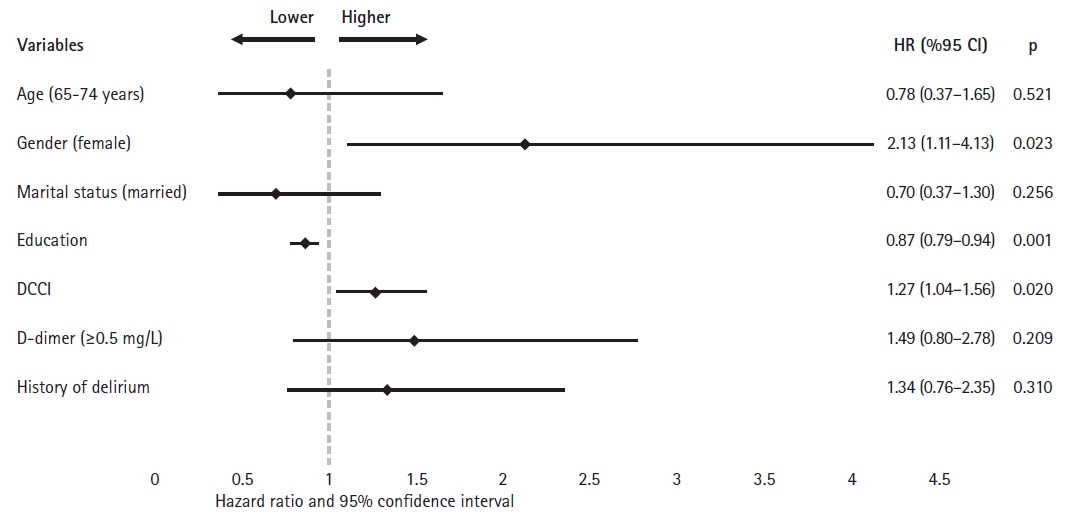
Multivariate analysis of the relationship between cognitive impairment and clinical variables with the respective adjusted hazard ratios. DCCI, Deyo/Charlson Comorbidity Index Score.
In the univariate logistic regression analysis, depression (odds ratio [OR]=2.33; 95% CI 1.12–4.86; p=0.024) and anxiety (OR=3.94; 95% CI 1.75–8.89; p=0.001) symptoms at the end of follow-up were associated with reduced cognitive performance. However, in the multivariate analyses, the associations remained insignificant (OR=0.83; 95% CI 0.26–2.60; p=0.752 and OR=2.15; 95% CI 0.63–7.30; p=0.220, respectively).
DISCUSSION
The most important finding of this study, and also the answer to our first hypothesis, was that, on average, almost half of the survivors showed reduced cognitive performance 15 months after hospitalization for COVID-19. Regarding our second study question, female sex, comorbidities, and low educational level were important predictors of reduced cognitive performance after adjusting for confounding factors. Contrary to previously described pathophysiological mechanisms,13,14,19,20) reduced cognitive performance was independent of baseline blood inflammatory marker levels and mood status on mental assessment at the end of follow-up.
Information on cognitive and mental health post-COVID-19 is increasing. Recent studies have reported differing incidences of reduced cognitive performance in the early phase of COVID-19 ranging between 61.5% and 80%.34) Furthermore, prospective cohort studies have examined the impact of long COVID syndrome on cognition at various time intervals.5,6,34-38) Reduced cognitive performance ranged from 23% to 65% in studies examining mental health 3–6 months after recovery from COVID-19.5,6,37) A meta-analysis of 10,530 participants with a mean age of 52 years showed that memory problems varied from 22% to 35%.35) That study also observed a higher prevalence of cognitive impairment at 6 months than during the first 3 months.35) At the 1-year follow-up after discharge, the reported prevalence of reduced cognitive performance is between 21.2% and 49.1% in patients with a mean age <60 years.37,38) In addition, the incidence of reduced cognitive performance was 12.5% in individuals aged ≥60 years and older examined 1 year after discharge.36) No research has assessed cognition in older adults at >1 year after recovering from COVID-19. The results of the present study indicated a higher prevalence of reduced cognitive performance in the long-term follow-up (44.3%). The differences in results across studies are most likely owing to sociodemographic, clinical, and methodological differences as well as the timing of cognitive function assessment after acute infection. Our findings extend those of previous studies by demonstrating the persisting prevalence of reduced cognitive performance in cognitively frail older individuals 15 months after discharge. However, the predictors of reduced cognitive performance in long-term COVID syndrome and whether this condition is permanent remain unclear.
Comorbidities, female sex, and a low level of education were predictors of long-term cognitive impairment in our study. Similar to our findings, a large-scale web-based study of 81,337 participants reported a link between comorbidities and reduced cognitive performance in individuals who had recovered from COVID-19.39) In contrast, another study by Miskowiak et al. observed a mean DCCI score of 2.9 and reported no association between comorbidity and cognitive status after recovery.6) One possible reason for this difference may be that their study was conducted in a younger population (mean age, 56.2 years) and had fewer participants (n=29). Consistent with our results, a study with a small sample size reported that women had a 7.35-fold increased risk of subjective decline in cognitive status 5 months after discharge.5) A prospective online survey conducted 12 months after recovery found that female sex showed borderline significance for cognitive impairment risk.38) The Atahualpa cohort study from Ecuador reported no female predominance in reduced cognitive performance at 6 months in individuals recovering from mild symptomatic COVID-19 infection.40) The reason why female sex emerged as a negative factor in our study may be because men tend to have more severe COVID-19 than women.41) At the beginning of the study, individuals experiencing more severe conditions may not have been included because they failed to meet the inclusion criteria. Those experiencing more severe conditions may exhibit a greater decline in cognitive performance, possibly owing to inflammatory processes. Therefore, women may have demonstrated a higher incidence of cognitive performance decline in our study. However, further research that incorporates inflammatory parameters is required for a more objective assessment of these differences. Given the impact of education on cognitive function in old age, it is not surprising that we observed an increased risk of long-term reduced cognitive performance with decreasing educational level.42) Education is associated with long-term cognitive performance.43,44) The number of formal years of education completed by individuals is positively associated with cognitive function throughout adulthood and predictive of a lower risk of dementia in later stages of life.45) A study conducted in China showed a decreasing risk for each year of education (β=-0.098, p=0.013), whereas a study in New York showed that an education level of <12 years was a significant risk factor for long-term reduced cognitive performance after COVID-19 (OR=5.21; 95% CI 2.25–12.09).44) Thus, more research is needed to identify, the risk factors for long-term cognitive decline related to COVID-19. In addition to studies evaluating risk factors, further studies using intermittent testing are needed to determine whether this cognitive decline is permanent. In this context, a prospective study assessing cognitive decline and risk factors in individuals whose cognitive tests were objectively assessed before the COVID-19 pandemic who did and did not develop COVID-19, and who are matched for education and comorbidities would be very valuable.
Mood disorders can be a cause and consequence of reduced cognitive performance; however, the presence of this relationship following COVID-19 remains controversial.19,20) A cross-sectional study of 153 participants hospitalized for COVID-19 reported that depression was associated with worse cognitive health 3 months after illness.46) However, one limitation of this study was that it did not examine cognitive complaints using formal screening tools. Consistent with our results, a recent study reported no significant correlation between most cognitive tasks and depression and anxiety scales, whereas cognitive tests showed a statistically significant correlation with a very small effect (percentage of variance <15%).47) Another prospective study did not observe a correlation between depression, anxiety, and cognition, suggesting that mood disorders may not lead to reduced cognitive performance.44,48) We extend these findings by showing them in the long term and isolated older adults. However, the mechanisms underlying the negative effects of COVID-19 on cognition remain unclear. Given the negative effects of depression on cognition, one reason that we did not observe this effect in our multivariate analyses may be the complex relationships among many existing variables, which remain to be elucidated. In addition, although evidence suggests a relationship between serum inflammatory markers in the course of acute COVID-19 illness and reduced cognitive performance in the early period, we did not find any impact of these markers on future reduced cognitive performance.6)
Post-intensive care syndrome, which manifests as the loss of cognitive and physical functions in patients who survive intensive care, may be responsible for some cognitive impairments caused by COVID-19.15,17,18) In our study, we observed no differences in cognitive function among patients who survived intensive care. Similarly, a study in Belgium also observed no such difference49); however, a cohort study conducted in New York reported that post-intensive care syndrome was common in patients who survived COVID-19.15,17)
The mechanisms underlying cognitive sequelae after COVID-19 remain unclear.34) SARS-CoV-2 with neuro-invasion and neurotropism may cause patients to develop delayed neurodegenerative diseases.14) Transsynaptic transmission of the virus to the limbic structures and deeper parts of the central nervous system could explain the occurrence of neurological symptoms.14) Furthermore, reduced cognitive performance may be associated with hyperinflammation, hypoxia, and thrombotic events in the central nervous system.13,14) Whole-brain cortical and hippocampal atrophy, hypoxic-ischemic brain changes, and cerebral small-vessel disease are neuropathological events that develop owing to inflammatory reactions and oxidative stress related to this infection.35) We did not design the present study to investigate these pathophysiological mechanisms. However, the results demonstrated that cognitive impairment may occur during long-term follow-up, although it is not yet clear by what mechanism.
This study has some limitations. First, the lack of information on comprehensive mental examinations of participants before infection is a common limitation shared with similar studies.5,6,36-38) To minimize selection bias while forming the study cohort, we used strict exclusion criteria (e.g., participants who had not been diagnosed with a cognitive disorder, had never taken medication for dementia, or had no limitations in daily and/or instrumental life activities). Second, the participants’ cognitive function was assessed using a telephone-based screening tool to decrease the spread of COVID-19. Difficulties exist in performing cognitive tests on phones. Particularly in the older population, individuals may have serious hearing problems and tend to give more superficial answers to questions compared to face-to-face interviews. This makes it difficult to obtain accurate results. Therefore, we did not include patients with severe hearing loss in our study and used a telephone cognitive test whose validity and reliability have been confirmed in a Turkish population.23) Finally, some risk factors that may affect cognitive status may have been overlooked.
In conclusion, the results of this study provided evidence that older adults discharged following hospitalization for COVID-19 were at risk for long-term cognitive impairment. In addition to the previously established short-term predictors, we identified female sex and comorbidity burden as important risk factors for the long-term development of reduced cognitive performance. Further studies are needed to develop potential therapeutic interventions after COVID-19 among older people at a higher risk for decreased cognition.
Notes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization, ED, MIN; Data curation, BGYV; Funding acquisition, BGYV; Investigation, ED, BGYV, MIN; Methodology, BGYV, MIN; Project administration, ED; Supervision, MIN; Writing-original draft, ED, BGYV; Writing-review & editing, ED, BGYV, MIN.