Frailty Screening and Detection of Geriatric Syndromes in Acute Inpatient Care: Impact on Hospital Length of Stay and 30-Day Readmissions
Article information
Abstract
Background
Frailty is prevalent in acute care and is associated with negative outcomes. While a comprehensive geriatric assessment to identify geriatric syndromes is recommended after identifying frailty, more evidence is needed to support this approach in the inpatient setting. This study examined the association between frailty and geriatric syndromes and their impact on outcomes in acutely admitted older adults.
Methods
A total of 733 individuals aged ≥65 years admitted to the General Surgery Service of a tertiary hospital were assessed for frailty using the Clinical Frailty Scale (CFS) and for geriatric syndromes using routine nursing admission assessments, including cognitive impairment, falls, incontinence, malnutrition, and poor oral health. Multinomial logistic regression and Cox regression were used to evaluate the associations between frailty and geriatric syndromes and their concomitant impact on hospital length of stay (LOS) and 30-day readmissions.
Results
Greater frailty severity was associated with an increased likelihood of geriatric syndromes. Individuals categorized as CFS 4–6 and CFS 7–8 with concomitant geriatric syndromes had 29% and 35% increased risks of a longer LOS, respectively. CFS 4–6 was significantly associated with functional decline (relative risk ratio =1.46; 95% confidence interval [CI], 1.03–2.07) and 30-day readmission (hazare ratio=1.78; 95% CI, 1.04–3.04), whereas these associations were not significant for CFS 7–8.
Conclusions
Geriatric syndromes in frail individuals can be identified from routine nursing assessments and represent a potential approach for targeted interventions following frailty identification. Tailored interventions may be necessary to achieve optimal outcomes at different stages of frailty. Further research is required to evaluate interventions for older adults with frailty in a wider hospital context.
INTRODUCTION
As the population continues to age, healthcare systems face new challenges in caring for the increasing number of frail older individuals. In acute care settings, the prevalence of frailty can range from 30% to 80%,1,2) with frailty at admission being linked to higher risks of mortality, disability, longer hospital stays, readmissions, and higher healthcare costs.3,4) In addition, older individuals may present with frailty-related geriatric syndromes and hospital-acquired complications such as falls, delirium, and functional decline, which can further contribute to poor patient outcomes.5)
Guidelines recommend assessing the presence of frailty, followed by the Comprehensive Geriatric Assessment (CGA), as the best practice for frailty management.6) While CGA remains a cornerstone in managing frailty, the available evidence on CGA centers on specific conditions in specialized wards or services such as acute care of the elderly (ACE) units or orthogeriatrics.7-9) Additionally, many of these studies neither measured frailty status or they implemented general nutritional and physical activity interventions to reduce overall frailty levels.10,11) Therefore, evidence presumed to be applicable for establishing acute care interventions for frail older persons is not derived from studies that stratified individuals based on their frailty status.12) While “front door” and acute frailty units show promise in incorporating CGA principles for managing frail older persons,13,14) further evidence is needed to support the systematic and wider use of frailty assessment and to demonstrate how frailty levels can risk-stratify and prompt identification of geriatric syndromes to guide CGA interventions.15,16)
Therefore, this study aimed to determine the associations between frailty status and the presence of geriatric syndromes among older individuals who were acutely admitted to the hospital and to assess the associations between frailty status and hospital length of stay (LOS) and 30-day readmissions in patients with geriatric syndromes. Examining the association and impact of frailty and geriatric syndromes in hospitalized older adults may inform the development of interventions and care pathways that utilize frailty status to target older adults for CGA in the acute inpatient setting.
MATERIALS AND METHODS
Study Population
We analyzed data from patients admitted to the Department of General Surgery registered in a clinical database, between January 1, 2019, and March 31, 2019. The database was designed to assess geriatric syndromes and frailty and comprised de-identified health-related data from electronic records, including demographics, in-hospital information, comorbidities, illness severity, and routine nursing assessments. We included individuals aged ≥65 years who were admitted to the General Surgery Service of the Emergency Department. The exclusion criteria were elective or same-day admissions for planned surgical procedures. The National Healthcare Group Domain Specific Review Board (DSRB) granted ethical approval for this study (DSRB Reference No. 2022/00578). Also, this study complied the ethical guidelines for authorship and publishing in the Annals of Geriatric Medicine and Research.17)
Data Collection
We collected baseline variables including age, sex, ethnicity, and comorbidities (weighted Charlson Comorbidity Index [CCI], a tool widely used to assess the severity of comorbidities), assigning weighted scores to 19 different comorbid conditions based on their potential to impact clinical outcomes, with scores assigned to indicate low, medium, high, and very high comorbidity burden categories.18) We also collected data on the modified Severity of Illness Index (SII), a four-level burden of illness measure validated in the local population of older adults, with excellent inter-rater agreement and predictive validity for adverse outcomes, including hospital LOS and cost of hospitalization.19) We assessed the outcome variables of hospital LOS and 30-day readmission following discharge from the index hospitalization.
We assessed frailty using the Clinical Frailty Scale (CFS), a global synthesis assessment tool consisting of a 9-point scale that allows classification across the frailty continuum from 1 (very fit) to 9 (terminally ill).20) The CFS is a well-validated measure of frailty that has been shown to predict adverse outcomes in older adults, including mortality, institutionalization, and functional decline. At our institution, trained nurses routinely rate the CFS based on a previously published approach21) in patients aged ≥65 years who are triaged as non-P1 (highest acuity) cases upon admission to the Emergency Department.
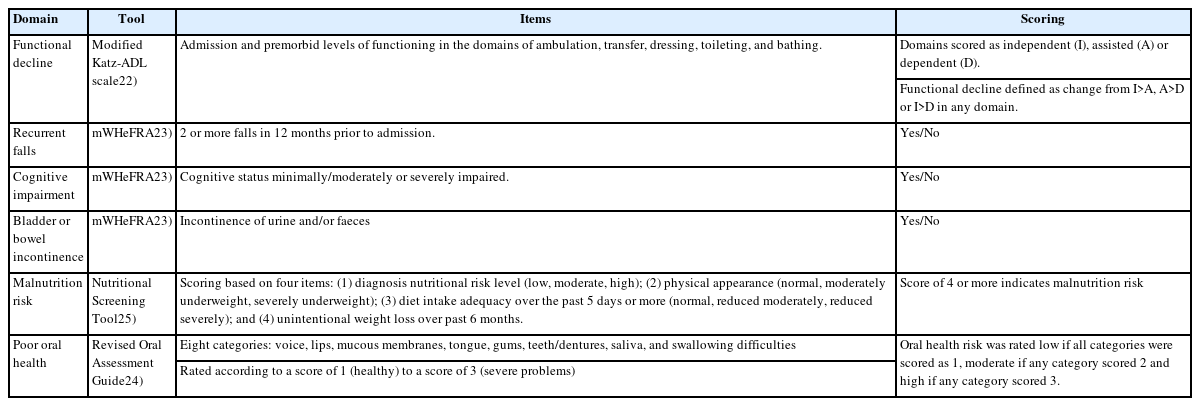
We used data from routine nursing assessment tools performed by registered nurses for all patients within 24 hours of ward admission to identify geriatric syndromes, including functional decline, recurrent falls, cognitive impairment, poor oral health, bladder or bowel incontinence, and malnutrition risk. Functional decline was defined as any change in activities of daily living (ADL) status at admission compared to the premorbid status based on a modified Katz-ADL scale consisting of feeding, dressing, bathing, toileting, transferring, and ambulation.22) To assess recurrent falls, we used a specific item from the modified Western Health Falls Risk Assessment Tool (mWHeFRA) to identify any history of two or more falls in the past 12 months.23) Next, we assessed cognitive impairment and bladder or bowel incontinence using specific items from the mWHeFRA. We also assessed poor oral health using the Revised Oral Assessment Guide (ROAG)24) and malnutrition risk using the Nutritional Screening Tool (NST), a locally validated nutrition risk screening tool developed for hospitalized older adults.25) A summary of the items used to identify geriatric syndromes is shown in Table 1.
Statistical Analysis
We described categorical variables as absolute numbers and corresponding percentages, and continuous variables as means with standard deviation or medians with interquartile range (IQR) for non-parametric data.
To analyze the association between frailty status and the presence of geriatric syndromes, we stratified the CFS levels into CFS 1–3, 4–6, and 7–8 categories. We analyzed the relationships between baseline variables and geriatric syndromes with CFS categories using one-way analysis of variance or the Kruskal–Wallis test for continuous variables and the chi-square test for categorical variables. We then performed multinomial logistic regression to evaluate CFS levels as predictors of the presence of geriatric syndromes, both unadjusted and adjusted for age, sex, ethnicity, comorbidities, and illness severity, using CFS 1–3 as the reference group.
To determine the association between frailty and concomitant geriatric syndromes and the outcomes of LOS and 30-day readmission, we calculated hazard ratios for the time to discharge and 30-day readmission using multivariable Cox regression adjusted for age, sex, ethnicity, illness severity, and comorbidities, using the non-frail (CFS 1–3) or those without any geriatric syndromes as the reference group. The proportional hazard assumption was verified and met using Schoenfeld residuals.
To account for missing data, we conducted multiple imputations using chained equations.26) Missing values for CFS, weighted CCI, functional decline, mWHeFRA, ROAG, and NST were imputed. We generated 30 different datasets and pooled the coefficients. As a sensitivity analysis, we also performed a complete case analysis, excluding individuals with missing values. Missing data are reported in Supplemental Table S1. Statistical significance was set at p<0.05. Statistical analyses were performed using Stata version 13.0 (StataCorp LLP, College Station, TX, USA).
RESULTS
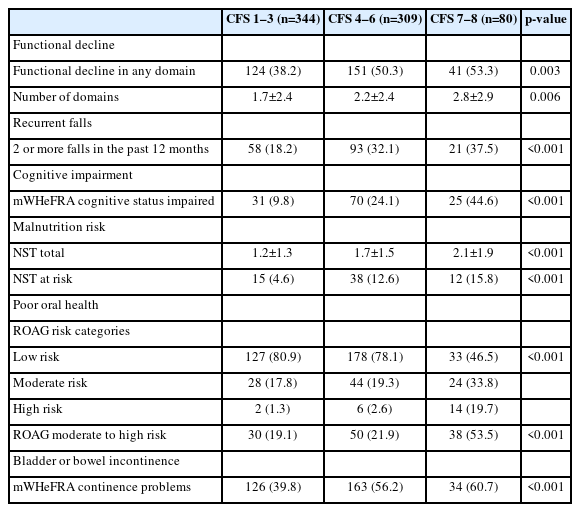
Among 750 eligible individuals admitted during the study period, 733 (97.7%) had available CFS data. The mean age of the included individuals was 77.6±8.2 years, half were female and most were of Chinese ethnicity. Table 2 shows the baseline characteristics of the study population according to the CFS categories. Among the 733 included individuals, 344 (45.9%), 309 (41.2%), and 80 (10.7%) were classified as CFS 1–3, CFS 4–6, and CFS 7–8, respectively. Individuals who were frailer were older and had a greater comorbidity burden, with no differences in illness severity on admission across frailty levels.
Association of Frailty with Geriatric Syndromes
We observed an increasing frequency of geriatric syndromes with greater severity of frailty. Specifically, the proportion of individuals with functional decline on admission, recurrent falls, cognitive impairment, malnutrition risk, and poor oral health was significantly higher in those with higher levels of frailty (Fig. 1, Table 3).
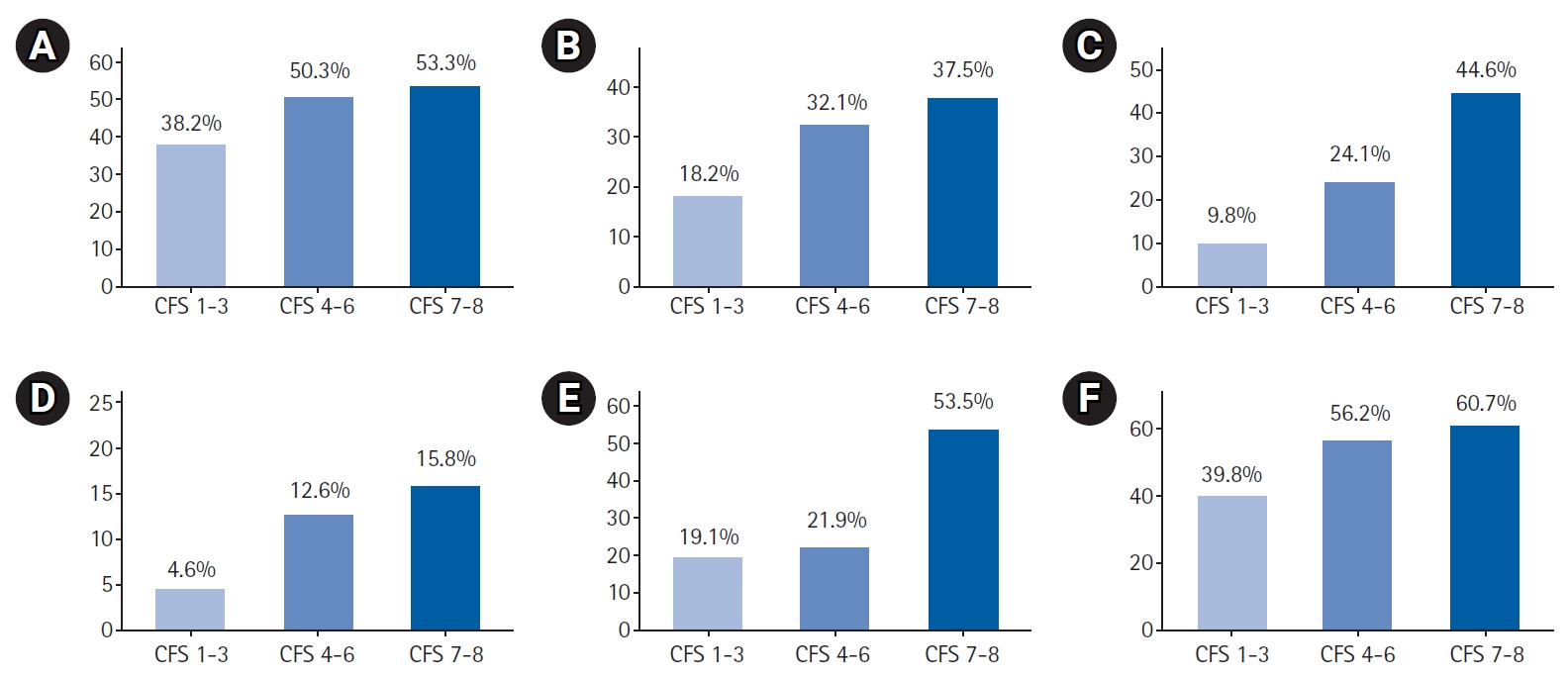
Geriatric syndromes by Clinical Frailty Scale (CFS) levels: (A) functional decline, (B) recurrent falls, (C) cognitive impairment, (D) malnutrition risk, (E) poor oral health, and (F) bladder or bowel incontinence.
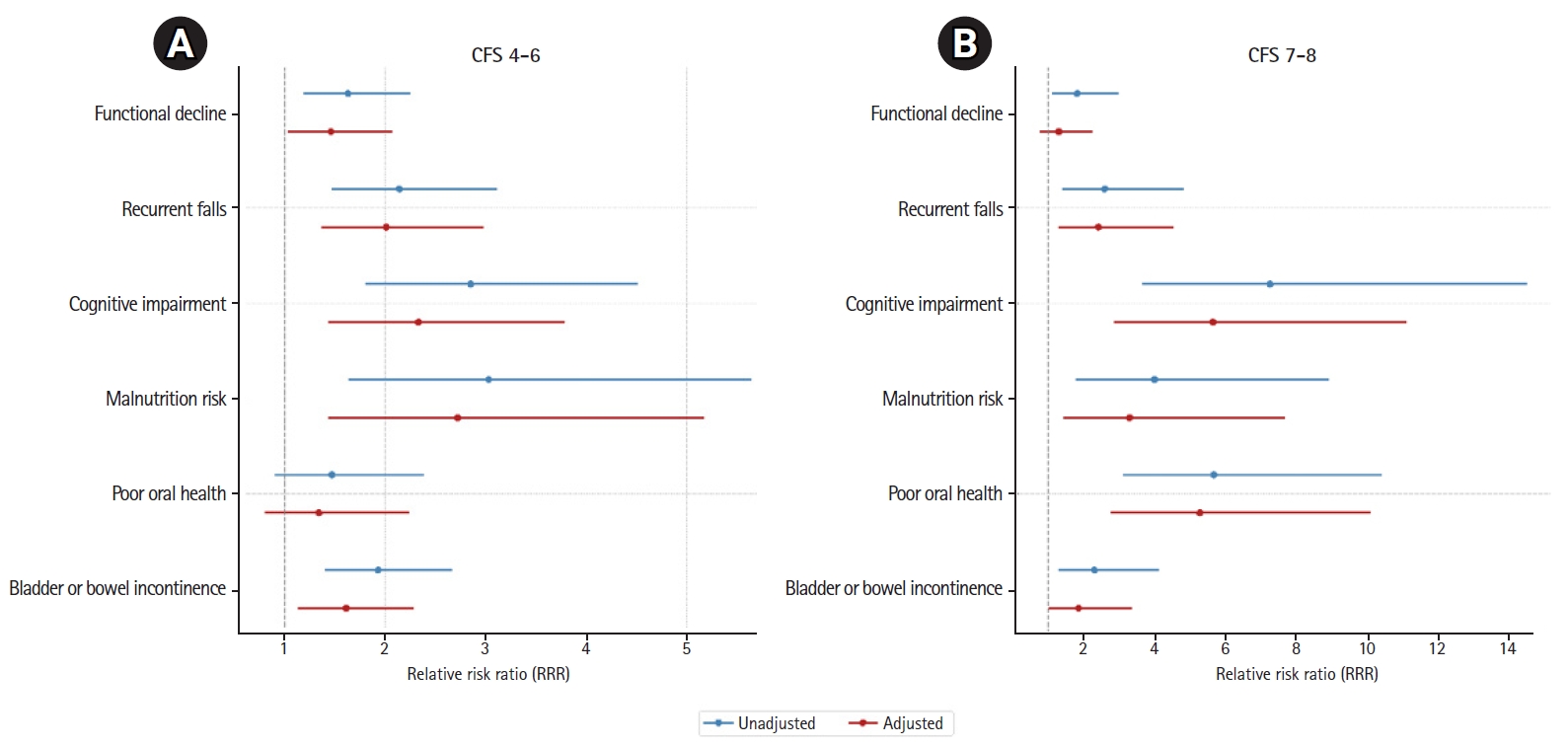
In both unadjusted and adjusted multinomial logistic regression models using relative risk ratios (RRRs), we observed increased risks of detecting geriatric syndromes of recurrent falls, cognitive impairment, malnutrition, and bladder or bowel incontinence for individuals in both the CFS 4–6 and CFS 7–8 categories, using the CFS 1–3 category as the reference group (Fig. 2, Supplemental Table S2). In adjusted analyses, individuals in the CFS 4–6 category had a significantly increased risk of functional decline (RRR=1.46; 95% confidence interval [CI], 1.03–2.07), but this increased risk was not observed in the CFS 7–8 category. In contrast, we observed an increased risk of poor oral health for individuals in the CFS 7–8 category (RRR=4.50; 95% CI, 2.40–8.44), but not in the CFS 4–6 category.
Impact of Frailty and Geriatric Syndromes on LOS and 30-Day Readmission Outcomes
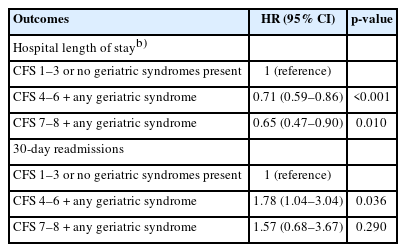
Hospital LOS increased with greater severity of frailty, with median OS increasing from 5.9 days (IQR 2–6), 8.1 days (IQR 2–9), and 8.3 days (IQR 3–8.5) across the CFS 1–3, CFS 4–6, and CFS 7–8 categories, respectively. In multivariate Cox regression analysis, increasing frailty with any concomitant geriatric syndrome was associated with a lower probability of discharge. Specifically, individuals in the mild-to-moderately frail and severely frail categories showed 29% and 35% reductions in the probability of discharge at any given LOS, respectively. The 30-day readmission rates were 11.6% (40 patients), 17.2% (53 patients), and 18.8% (15 patients) across CFS 1–3, CFS 4–6, and CFS 7–8 categories respectively. In multivariable Cox regression analysis, the hazards of 30-day readmission increased for individuals in the CFS 4–6 category, but not for those in the CFS 7–8 category (Table 4). In the sensitivity analyses, the associations determined through the complete case analysis demonstrated similar results to those obtained using the imputed data (Supplemental Tables S3, S4).
DISCUSSION
This study investigated the relationship between frailty and geriatric syndromes in acutely admitted older adults and their impact on hospital LOS and 30-day readmission. The results showed that frailty was associated with a greater likelihood of geriatric syndromes, including functional decline, recurrent falls, cognitive impairment, malnutrition risk, incontinence, and poor oral health. However, severe frailty (CFS 7–8) was not associated with functional decline. Additionally, greater frailty severity in the presence of geriatric syndromes was linked to increased LOS, but increased risk of 30-day readmissions was only significantly associated with mild-to-moderate frailty (CFS 4–6), and not with severe frailty. Overall, our results underscore the potential of frailty identification in flagging the possible presence of geriatric syndromes, and that frailty with concomitant geriatric syndromes is associated with poorer outcomes, with these outcomes varying depending on the level of frailty.
While CGA-based multidisciplinary care in inpatient settings has demonstrated beneficial effects, outcomes vary depending on the clinical setting and model adopted.9) Positive outcomes include an increased likelihood of individuals being alive and in their own homes at follow-up and reduced institutionalization rates. However, the effects on mortality, dependence, and healthcare costs have been inconsistent.27) In another meta-analysis, CGA was effective in improving quality of life and reducing caregiver burden but did not affect the hospital LOS.28) Moreover, evidence for the benefits of CGA is setting-specific, differing by ward- or team-based models of care as well as by specific conditions such as oncology29) or perioperative care,30) while most studies utilize age-based inclusion criteria.9) Although chronological age and specific conditions have traditionally guided clinical decision-making, our findings suggest that frailty is an indicator of an elevated risk of poor health outcomes in the inpatient setting. With the identification of frailty, emerging evidence supports the introduction of structured exercise programs and nutritional modifications targeting hospitalized frail older adults.31)
The CFS was originally introduced as a means of summarizing the results of the CGA, which is typically conducted in specialized geriatrician-led settings. Considering the increasing number of older adults accessing healthcare services, frailty screening is being used as a risk stratification approach in wider hospital settings.32) This approach uses frailty level as a triage tool to recognize geriatric syndromes in at-risk individuals and trigger referrals for CGA and its associated interventions.33,34) Additionally, integrating frailty assessments into routine care adds value by guiding clinicians to develop more rational, person-centered care plans that recognize under-detected geriatric syndromes, and prioritize achieving functional goals beyond treating individual diseases alone.35,36)
Our results revealed that, with an increase in frailty levels across the three CFS categories, the likelihood of detecting geriatric syndromes also increased. A notable exception was in the domain of functional decline, where we observed a significant increase in the risk of functional decline among individuals in the mild-to-moderately frail (CFS 4–6) category but not for those in the severely frail (CFS 7–8) category. This finding could be due to a higher baseline level of functional impairment in patients with more severe frailty upon admission, making changes in functionality during hospitalization less discernible.
Previous studies have also emphasized the predictive utility of individual and combined indicators of geriatric syndromes for healthcare utilization.37,38) Frailty, dementia, and acute confusion predict prolonged LOS, delayed discharge, institutionalization, and 30-day readmission.5) In a systematic review and meta-analysis, greater frailty severity was common in older patients with unplanned hospital admissions and was associated with increased risks, including mortality and longer LOS. However, moderate-to-severe frailty levels were inconsistently related to 30-day readmissions,39) an observation similar to that in our study, in which the presence of geriatric syndromes did not fully account for 30-day readmissions in severely frail patients. While few studies have focused on the readmission risk in severely frail individuals, potentially modifiable risk factors such as medication management and care coordination may influence outcomes in these individuals.40)
Our finding of a lower likelihood of detecting bladder or bowel incontinence in severely frail individuals than in mild-to-moderately frail individuals may be explained by specific items in the mWHeFRA continence domain, where participants with indwelling urinary catheters are scored as zero, denoting a low risk of incontinence. Moreover, the mWHeFRA ascribes higher risk scores to individuals with urinary frequency, urgency, and nocturia, which may not be apparent in severely frail, functionally impaired patients. These findings indicate the need to refine or utilize screening questionnaires to more accurately detect geriatric syndromes. Nevertheless, our results highlight the potential for using frailty levels to predict the likelihood of geriatric syndromes, with the potential to tailor interventions to meet individual needs.41,42) For example, at advanced stages of frailty, strategies to promote advanced care planning43) or pain and symptom management may be more relevant than focusing on geriatric syndromes alone.44)
Our findings also highlight the potential of utilizing routinely collected admission information to screen for geriatric syndromes rather than introducing new tools that may require additional resources, expertise, and time.45) Although not all the items used to identify potential geriatric syndromes were validated as syndrome-specific screening tools, our findings indicate that such an approach may still be beneficial for identifying these geriatric conditions. Utilizing existing data sources may avoid the introduction of additional processes into the healthcare system and minimize the burden on healthcare providers while enabling the extension of geriatric care beyond specialized geriatrician-led settings and facilitating the implementation of routine geriatric screening in hospitals.46) Nevertheless, further studies are necessary to confirm the presence or absence of geriatric syndromes using this approach.
The strengths of this study include the assessment of frailty and geriatric syndromes in hospitalized older adults using standardized measures. In addition, we compared the results from multiple imputations and complete case analyses to address missing data. However, this study has several limitations. First, we identified geriatric syndromes using routinely collected data from nursing admission assessments, which may not have captured all the relevant syndromes. CGA is a multidimensional, interdisciplinary diagnostic process that evaluates an older adult's medical, functional, cognitive, and psychosocial status. Other domains, including social need assessments and discharge planning, are required to develop and implement coordinated care plans that address these issues. Second, although we identified geriatric syndromes through screening tools, confirmation of the presence of geriatric syndromes by a geriatrician or formal diagnosis was not available. Further studies exploring the addition of screening for other CGA domains, such as polypharmacy, sensory impairment, and confirmatory diagnosis of syndromes, such as dementia or delirium, are needed. Third, information on frailty and geriatric syndromes was obtained on admission and within 24 hours of admission; thus, we were unable to account for geriatric syndromes that could have developed during admission. As our database was primarily structured to collect data on geriatric syndromes and frailty, information on other variables such as surgical diagnoses, type of surgery, and complications was not available. While our study did not demonstrate differences in the severity of illness at admission between the CFS groups, further studies including more details on intervening events for the analysis of longitudinal outcomes are recommended. Finally, these results cannot be generalized to other inpatient settings and disciplines.
Despite these limitations, our findings suggest the potential role of routine frailty assessment in identifying geriatric syndromes in acute inpatient settings. Our findings also indicate that the presence of geriatric syndromes in patients with severe frailty may not affect 30-day readmission, suggesting that other factors may influence this outcome. Additionally, routine, existing nursing admission assessments for geriatric syndrome screening could be a practical approach to facilitate the extension of geriatric care and trigger CGA beyond specialized geriatrician-led settings to reach older adults across hospitals. Further research should focus on developing and implementing feasible CGA interventions to address the complex needs of frail older adults in acute-care settings.
Notes
The authors wish to thank all participants in the study and Dr Glenn Tan and the Department of General Surgery, TTSH for their support.
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization, JC; Data curation, JC, JQC, KKK, JKF; Investigation, JC; Methodology, JC, SYC; Supervision, HNT; Writing-original draft, JC; Writing-sreview & editing, JC, CL, SYC, HNT.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4235/agmr.23.0124.
Missing values for variables studied
Multinomial logistic regression for the association between Clinical Frailty Scale (CFS) levels and geriatric syndromes (imputed data)
Multinomial logistic regression for the association between Clinical Frailty Scale (CFS) levels and geriatric syndromes (complete case analysis)
Cox proportional hazards models: associations of frailty levels and any concomitant geriatric syndrome with hospital length of stay and 30-day readmissions (complete case analysis)a)