Anticholinergic Cognitive Burden as a Predictive Factor for In-hospital Mortality in Older Patients in Korea
Article information
Abstract
Background
With the increasing prevalence of chronic disease due to aging, many older adults experience multimorbidity and polypharmacy. Medications with anticholinergic properties are particularly associated with adverse cognitive outcomes, including functional decline and mortality. We assessed the clinical impact of anticholinergic cognitive burden (ACB) on clinical outcomes of older patients acutely admitted to a single, hospitalist-operated medical unit of a tertiary hospital in Korea.
Methods
This retrospective study reviewed electronic medical records of 318 patients aged 65 years or older admitted to the hospitalist-operated medical unit through the emergency department of Seoul National University Hospital. The analyzed clinical outcomes were the length of hospital stay, in-hospital mortality, unplanned intensive care unit admission, and unexpected readmission within 30 days.
Results
The clinical outcomes did not differ between patients who took five or more drugs and those who did not. Patients with an ACB score of 3 or higher had a higher in-hospital mortality rate and longer hospital stay than those who did not. After adjusting for confounding factors, an ACB score of 3 or higher was an independent predictive factor for in-hospital mortality (odds ratio=3.09; 95% confidence interval, 1.18–8.06).
Conclusion
ACB rather than the number of medications was associated with in-hospital mortality in acutely ill older patients. Further analytic and interventional studies are required to assess potentially inappropriate medication use and ACB in older inpatients.
INTRODUCTION
With the increasing prevalence of chronic disease with aging, many older adults are treated concurrently for two or more diseases, a condition commonly referred to as a state of multimorbidity.1,2) A recent report indicated that the prevalence of multimorbidity in Korea is up to 73%,3) predominantly due to common diseases such as hypertension, osteoarthritis, and hyperlipidemia. Since medical management for these conditions requires medications for specific diseases, older adults with multimorbidity are likely to take multiple medications simultaneously. Consequently, polypharmacy, a geriatric condition defined as taking multiple medications (usually five or more per day), is a frequently encountered clinical condition in medical care for older adults.4,5)
Medical care for older patients, especially those with polypharmacy, should consider factors such as prescribing cascade, drug-drug interactions, drug-disease interactions, and potentially inappropriate medications (PIMs) for older adults.6-9) Among these factors, the presence of PIMs is reportedly associated with increased adverse outcomes, including delirium, falls, functional decline, and mortality. Therefore, guidelines have recommended to reduce the use of or to replace PIMs with safer alternatives.8-10) Moreover, the concept of deprescribing, an individualized therapeutic strategy that considers the risks and benefits of medications according to patient functional and comorbid status, has emerged with efforts to minimize adverse outcomes with polypharmacy.11)
PIMs include a wide range of medications with anticholinergic properties affecting the cognitive states of older patients.8,12) Clinical evidence has shown associations between these medications and adverse cognitive outcomes,13) backed by evident scientific knowledge of the importance of acetylcholine signaling in cognitive performance.14,15) Accordingly, varying clinical measures to quantify anticholinergic cognitive burden (ACB) have been developed and validated.13,16,17)
Older patients admitted through the emergency department in tertiary hospitals tend to have multimorbidity and polypharmacy, suggesting high exposure to anticholinergic medications in this population that may lead to adverse outcomes.18,19) However, to our knowledge, no study has focused on ACB in acutely admitted older patients in Korea. Therefore, we assessed the clinical impact of ACB on clinical outcomes of older patients admitted via the emergency department of a single, hospitalist-operated medical unit of a tertiary hospital in Korea.
MATERIALS AND METHODS
Clinical Setting and Study Design
This retrospective study reviewed the electronic medical records at Seoul National University Hospital. We first searched for patients discharged from the hospitalist-operated medical unit at Seoul National University Hospital between February 2018 and October 2019. Among these patients, we included those 65 years of age and older admitted through the emergency department. To focus on acutely ill patients, we excluded patients admitted from the outpatient department and who were transferred from other wards, including the intensive care unit (ICU).
This study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital (No. H-1911-089-1079), which waived informed consent due to the retrospective nature of the study.
Measurement of ACB
Regular medications were assumed to be all the drugs regularly taken by each patient before hospitalization. We collected data on regular medications from each patient to check for polypharmacy and assessed the ACB by reviewing the patients’ regular medications and those prescribed during hospitalization. Each patient’s ACB score was calculated by summing the score according to the anticholinergic cognitive burden scale.17)
Data Collections and Outcome Measures
Demographic data such as sex and age and data on medical history were collected to calculate the Charlson Comorbidity Index (CCI). To assess the condition severity at the time of admission, vital signs and laboratory test results were also collected. Length of stay (LOS), in-hospital mortality, unplanned ICU admission, and unexpected readmission within 30 days were analyzed as clinical outcomes.
Statistical Analysis
Data are expressed as mean±standard deviation (SD) or numbers (percentage) unless stated otherwise. Chi-square or Fisher exact tests were used to compare categorical variables, while continuous variables were compared by Student t-tests. Univariate logistic regression was used to identify factors significantly influencing clinical outcomes. Multivariate logistic regression was performed with those factors to determine the independent predictive factors of ward mortality and delirium. Two-tailed p-values less than 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline Characteristics and Clinical Outcomes in the Study Population
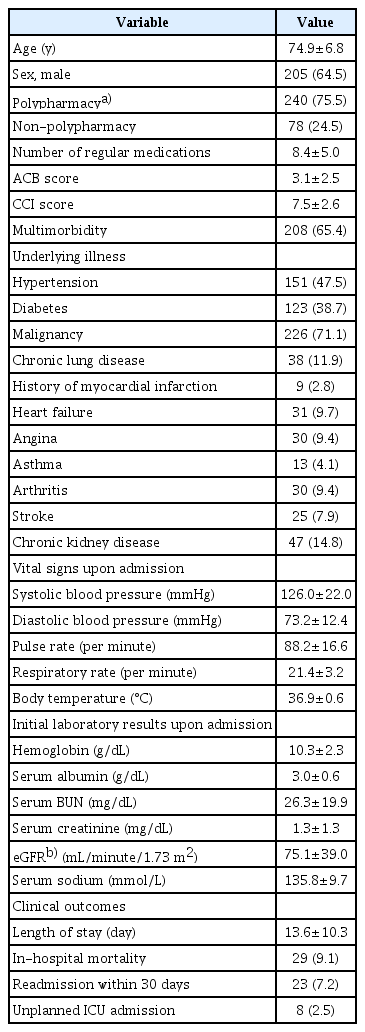
This study included 318 patients. Among them, the mean age was 74.9±6.8 years and 205 patients (64.5%) were men. A total of 240 patients (75.5%) were taking five or more drugs and the mean ACB score was 3.1 points. The proportions of patients with hypertension, diabetes, and malignancy were 47.5%, 38.7%, and 71.1%, respectively. Multimorbidity, defined as the co-existence of two or more chronic illnesses, was present in 208 patients (65.4%) and mean the CCI was 7.5±2.6 points. Regarding clinical outcomes, the in-hospital mortality and readmission rates within 30 days were 9.1% (29 patients) and 7.2% (23 patients), respectively. The mean LOS in the study population was 13.6±10.3 days. Finally, 8 patients entered the ICU unexpectedly (Table 1).
Comparisons of Patients according to the Number of Concurrent Regular Medications
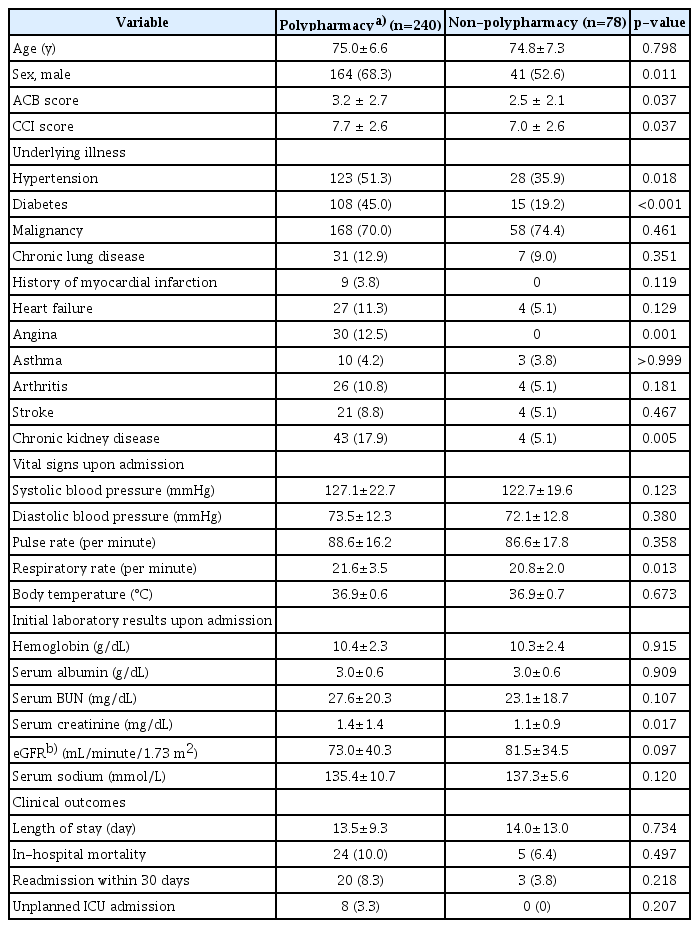
Patients taking five or more regular medications were categorized into the polypharmacy group. Age did not differ between the two groups. The proportion of men was higher in the polypharmacy group than that in the non-polypharmacy group (68.3 vs. 52.6%; p=0.011). Mean ACB and CCI score were higher in the polypharmacy group than those in the non-polypharmacy group (3.2±2.7 vs. 2.5±2.1 and 7.7±2.6 vs. 7.0±2.6, respectively). More patients in the polypharmacy group had hypertension, diabetes, angina, and chronic kidney disease. Admission vital signs except for respiratory rate were similar between groups. Serum creatinine level on admission was higher in the polypharmacy group, although blood urea nitrogen (BUN) level and estimated glomerular filtration rate (eGFR) did not significantly differ between the two groups. No significant differences in clinical outcomes were observed between the two groups for in-hospital mortality, readmission rate, mean LOS, and unexpected ICU admission (Table 2).
Comparisons of Patients according to ACB
High ACB, defined as an ACB score of 3 or higher, was observed in 156 patients (49.1%). Patients with high ACB scores were taking more concurrent regular medications than patients without high ACB scores (mean number of medications, 9.0±5.0 vs. 7.8±5.0; p=0.028). Chronic kidney disease as an underlying illness was more common in patients with high ACB score. There were no differences in age, sex, CCI score, or admission vital signs between groups. Patients with high ACB score had lower serum albumin levels. Patients with an ACB score of 3 or higher also showed a higher in-hospital mortality rate (14.1 vs. 4.3%; p=0.002) and longer hospital stays (mean LOS, 16.2±11.6 vs. 11.2±8.2 days; p<0.001) than those who did not. No differences in the proportions of readmission within 30 days or unplanned ICU admissions were observed between groups (Table 3).
Anticholinergic Burden as an Independent Predictive Factor for In-hospital Mortality
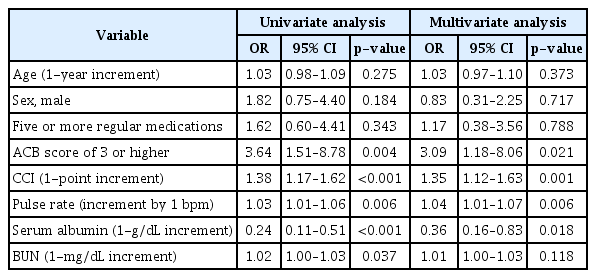
Univariate regression analyses for all variables revealed factors such as CCI score, high ACB, admission pulse rate, and serum albumin and BUN level on admission to be associated with in-hospital mortality. Age, sex, or five or more regular medications did not predict in-hospital mortality. After adjusting for confounding factors, an ACB score of 3 or higher remained an independent predictive factor for in-hospital mortality (odds ratio [OR]=3.09; 95% confidence interval [CI], 1.18–8.06). Moreover, one-point increment in CCI score (OR=1.35; 95% CI, 1.12–1.63), one beat per minute increment in pulse rate (OR=1.04; 95% CI, 1.01–1.07), and 1-g/dL increment in serum albumin level (OR=0.36; 95% CI, 0.16–0.83) were also associated with in-hospital mortality (Table 4).
DISCUSSION
In this study, we found that older patients admitted via the emergency department had a high prevalence of polypharmacy and were also heavily exposed to medications with anticholinergic properties. Both univariate and multivariate analyses revealed that ACB and not polypharmacy, per se, was associated with in-hospital mortality. To our knowledge, this is the first study to report the association between ACB and in-hospital mortality in acutely ill patients in Korea.
As the vast majority of commonly prescribed medications retain anticholinergic properties and also have biologic effects on cognitive performance, studies have evaluated the associations between anticholinergic exposure and clinical outcomes in older patients. Although long-term anticholinergic exposure and cognitive decline have been reported, controversies remain regarding the relevance of the short-term outcomes of ACB. Although a large-scale study showed an association between anticholinergic exposure and 2-year mortality,20) studies on in-hospital mortality indicated no definite adverse effect of anticholinergic exposure.21,22) In our study, ACB remained a significant predictor of mortality even after adjusting for comorbidity burden and polypharmacy.
Several possible mechanisms may explain the relationship between ACB and in-hospital mortality. Delirium, an important and preventable geriatric condition in hospitalized older adults, might be a mediator, as shown in studies on anticholinergic exposure, delirium, and mortality. For instance, a study from an acute care hospital in Canada reported delirium severity to be associated with a clinical-rated anticholinergic score.23) Another study from the United States including patients receiving palliative care showed a similar association between anticholinergic exposure according to an anticholinergic risk scale and delirium incidence.24) Both short-term and long-term time associations between delirium and mortality risk have been demonstrated.25,26) Unfortunately, as a retrospective study, we did not include delirium as a study variable because medical record review may fail to capture hypoactive delirium, which is reportedly worse in terms of clinical outcome.
The population in the present study had a relatively higher prevalence of polypharmacy with substantial ACB compared to those in previous studies in other countries on older acute patients.22,27,28) There may be several explanations for this difference. Firstly, the study population in the present study was inpatients admitted to an acute unit of a top-tier hospital in Korea, with a predictably high comorbidity burden. Secondly, the concepts of anticholinergic medications and PIMs are relatively unrecognized in Korea. Although Korea is experiencing an extreme pace of population aging, the concept of geriatric medicine is rarely taught in medical schools.29) Thirdly, specialized or fragmented care for older multimorbid patients might contribute to the occurrence of prescribing cascades that often involve PIMs.30) While our retrospective, descriptive study cannot address the contributions of these factors that may affect PIM and ACB in older patients with multimorbidities, our findings underscore the need for further research on the current nationwide status of medication usage in older adults.
As a retrospective observation performed by medical record review, our study has several limitations. Since our observations were based on the medical records of patients admitted to a single, hospitalist-run medical unit in a tertiary hospital, the characteristics of the patients in our study are not generalizable to the older population nationwide in Korea. Furthermore, our study lacks important geriatric baseline parameters including frailty, cognitive function, and daily functioning, and relevant outcome variables of ACB such as delirium and falls. Similarly, the functional outcomes of patients after discharge were unavailable in this study. Based on the results of this hypothesis-generating study, our upcoming prospective study with an interventional arm deprescribing PIMs and minimizing anticholinergic burden will provide better answers on the mediating mechanisms between ACB and clinical adverse outcomes.
In conclusion, ACB but not polypharmacy was associated with in-hospital mortality in acutely ill older patients. We hope that the results of this study lead to further analytic and interventional studies on PIMs and ACB in older inpatients.
Notes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, HWJ; Data curation, SM, SL, SJH; Investigation JHL, HWJ, IYJ; Methodology. JHL, HWJ, IYJ, SJH; Project administration, HWJ; Supervision, SJH; Writing-original draft, JHL, HWJ; Writing, review & Editing, SJH.