Analyzing the Factors Associated With Nocturia in Older People in the United States
Article information
Abstract
Background
The risk factors of nocturia in older adults remain unclear. We aimed to investigate factors associated with nocturia using the National Health and Nutrition Examination Survey (NHANES) data.
Methods
Among 40,790 participants, 4,698 participants aged ≥65 years were included from the NHANES dataset between 2005 and 2012. A multivariate logistic regression analysis was performed to determine the odds ratio (OR) for nocturia. A subgroup analysis was conducted based on sex and underlying diseases.
Results
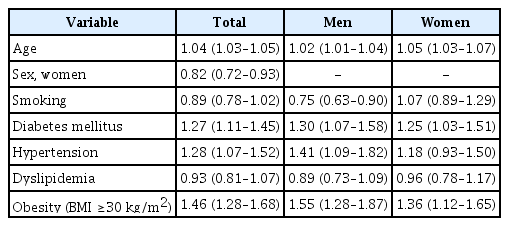
In the multivariate logistic regression model, obesity (OR, 1.46; 95% confidence interval [CI], 1.28–1.68), hypertension (OR, 1.28; 95% CI, 1.07–1.52), and diabetes mellitus (DM) (OR, 1.27; 95% CI, 1.11–1.45) were significantly associated with nocturia. These factors were associated with nocturia regardless of sex. In a subgroup of participants with hypertension, obesity (OR, 1.44; 95% CI, 1.25–1.67) and DM (OR, 1.26; 95% CI, 1.09–1.45) were associated with nocturia. In the additional analysis on patients with DM, nocturia was associated with obesity (OR, 1.33; 95% CI, 1.06–1.67) and duration of DM (OR, 1.02; 95% CI, 1.01–1.03).
Conclusion
This study demonstrated that hypertension, DM, and obesity were significantly associated with the prevalence of nocturia in older adult patients regardless of sex. In particular, obesity was associated with nocturia in every subgroup analysis.
INTRODUCTION
Geriatric disorders in older adults are often complex and atypical owing to multimorbidity and polypharmacy caused by several risk factors and chronic diseases.1–4) Geriatric syndrome is defined as a condition characterized by frequent onset of disease that results in a reduced quality of life due to deterioration of different functions and response to stimulation caused by multiple factors.1,2) Geriatric syndrome incurs disabling features in everyday life, thereby decreasing the quality of life in old age and increasing mortality in older people.5) Inouye et al.2) suggested the following as key disorders of geriatric syndrome: pressure ulcers, falls, functional decline, and urinary symptoms including urinary incontinence. Aside from urinary incontinence, the prevalence of other lower urinary tract symptoms (LUTS) is known to increase in older adults.
Nocturia is the most common LUTS.6–8) According to the Korean Community Health Survey, the prevalence of nocturia has rapidly increased with aging.8) More specifically, nocturia results in reduced quality of life and work productivity in older adults.8–14) Nocturia also increases the risk of falls and fractures by increasing the frequency of waking up to urinate during the night, which eventually results in an increased mortality rate.12–14) Considering the increasing aging population and an environment that requires a systematic approach and therapy for geriatric syndrome, the prevalence and risk factors of nocturia in older adults should be identified.
Therefore, this study aimed to confirm the prevalence of nocturia in older adults and identify its associated risk factors using the National Health and Nutrition Examination Survey (NHANES) data.
MATERIALS AND METHODS
Study Population
Data were collected from the NHANES dataset between 2005 and 2012. Written informed consent was obtained from all participants, and the survey was approved by the National Center for Health Statistics’ ethical review board. Participants aged ≤64 years and those with missing data (urologic questionnaire, anthropometric, or laboratory data) were excluded. Finally, 4,698 of 40,790 participants were included in this study.
Definition of Nocturia
A structured questionnaire was used to investigate nocturia. Study participants were deemed to have nocturia if their answer to the following question was “two or more”: “During the past 30 days, how many times per night did you most typically get up to urinate, from the time you went to bed at night until the time you woke up in the morning?”
Definition of Underlying Disease
Body mass index (BMI) was defined as weight in kilograms divided by height in meters squared (kg/m2). Obesity was defined as a BMI≥30 kg/m2 based on the World Health Organization criteria.15) Hypertension was defined as having at least one of the following conditions: self-reported current use of antihypertensive agents, average systolic blood pressure (BP) ≥140 mmHg, or average diastolic BP ≥90 mmHg. Diabetes mellitus (DM) was defined as having at least one of the following conditions: self-reported current use of hypoglycemic agents or insulin, fasting plasma glucose ≥126 mg/dL, or random glucose ≥200 mg/dL. Those with a fasting total cholesterol ≥240 mg/dL and those taking dyslipidemia medications were considered to have dyslipidemia.
Structural Analysis
For summary statistics, we present the mean with a 95% confidence interval (CI) or prevalence (%) according to the presence of nocturia. Continuous variables were assessed using a t-test, whereas categorical variables were assessed using Pearson chi-square test. Because several studies have reported that the prevalence of nocturia differs according to age, sex, obesity, and underlying diseases such as hypertension, DM, and dyslipidemia,8,10) we performed a multivariate logistic regression analysis with these variables to determine the odds ratios (ORs) for nocturia. In addition, we conducted a subgroup analysis based on sex and underlying diseases. All analyses were performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). A p-value of ≤0.05 was considered significant.
Ethical Considerations
The protocol of NHANES was approved by the National Center for Health Statistics Research Ethics Review Board (protocol numbers 98-12, 2005–06, and 2011–17). Written informed consent was obtained from each study participant before the survey.
RESULTS
Baseline Characteristics
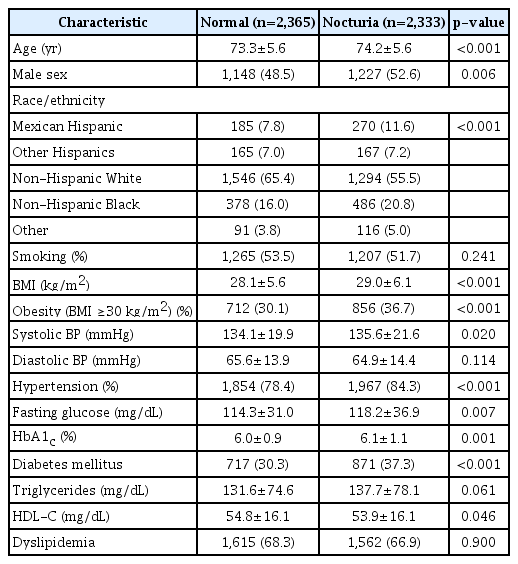
A total of 4,698 participants (2,375 men and 2,323 women) from the United States, aged 65–85 years (mean, 73.8 years), were examined. Among the included participants, 49.7% exhibited nocturia (men, 51.7%; women, 47.6%). The anthropometric, clinical, and biochemical characteristics of the participants based on the presence of nocturia are summarized in Table 1. Compared with the participants without nocturia, those with nocturia tended to be older, male, obese, hypertensive, or diabetic.
Factors Associated With Nocturia
In the multivariate logistic regression model, obesity (OR, 1.46; 95% CI, 1.28–1.68; Table 2), hypertension (OR, 1.28; 95% CI, 1.07–1.52) (Table 2), and DM (OR, 1.27; 95% CI, 1.11–1.45) (Table 2) were significantly associated with nocturia. These factors were associated with nocturia regardless of sex (Table 2). In a subgroup of participants with hypertension, obesity (OR, 1.44; 95% CI, 1.25–1.67) (Table 3) and DM (OR, 1.26; 95% CI, 1.09–1.45) (Table 3) were associated with nocturia. In the additional analysis on patients with DM, nocturia was associated with obesity (OR, 1.33; 95% CI, 1.06–1.67) (Table 3) and duration of DM (OR, 1.02; 95% CI, 1.01–1.03) (Table 3).
DISCUSSION
This study has confirmed the high prevalence of nocturia in older adults. Moreover, aside from age and sex, other factors (i.e., obesity, hypertension, and DM) were found to be associated with nocturia in a large population database.
A previous American study conducted in 2011 using the 2005–2008 NHANES data reported the prevalence of nocturia (urinating 2 or more times per night) in older men (≥75 years old) as 55.8%, similar to that in our study. The prevalence of nocturia in the younger population (aged 20–34 years) was 8.2%, lower than that in the older adults.16) In addition, as nocturia can result in symptoms (i.e., poor sleep, falls, and depression) that may act as risk factors of geriatric syndrome, its clinical importance should be highlighted.16)
Several previous studies have reported an association between obesity and nocturia.17) Although the complete mechanism underlying how obesity leads to nocturia is not well known, insulin resistance and hyperinsulinemia caused by obesity can stimulate the hypothalamic center that regulates sympathetic tone. This can increase the catecholamine level, which promotes prostate growth, resulting in nocturia caused by benign prostatic hyperplasia.18,19) In addition, pelvic atherosclerosis caused by obesity can induce deposition of ischemia-mediated altered collagen and bladder fibrosis, which induces urinary symptoms.20,21) Although the mechanism underlying the association of obesity with nocturia is not fully understood, several studies have reported an association between weight loss and improvements in nocturia. Breyer et al.22) reported that weight reduction improved nocturia at 6 months into a weight reduction program, compared with the obese women in the control group. In addition, another study on Korean women also reported that exercise improved nocturia in obese older women.23) Similarly, this study has confirmed the association between the onset of nocturia and obesity—regardless of sex, DM status, or hypertension status—using large-scale data, which strongly supports previous studies demonstrating an association between obesity and nocturia. Although the prevalence of obesity and nocturia among patients with type 2 DM was higher than that among those without type 2 DM, the association between obesity and nocturia remains controversial.24–26) Chung et al.24) reported that BMI was positively associated with nocturia in a Taiwanese study of patients with DM. In contrast, Chiu et al.25) showed that BMI was not associated with nocturia in another Taiwanese study of patients with DM. Furukawa et al. found a positive association between obesity and nocturia in older Japanese patients with DM but not in young and middle-aged patients.26) A subgroup analysis in this study confirmed that obesity is significantly associated with nocturia in patients with DM.
Previous epidemiological studies assessing the association between hypertension and nocturia have demonstrated a high prevalence of nocturia in patients using hypertensive medications.27) Hypertension can affect both glomerular filtration and tubular transport, resulting in increased urine volume.28,29) Furthermore, medication for hypertension (i.e., calcium channel blockers and beta blockers) has also been associated with nocturia.28,29) With reduced cardiac function due to hypertension, patients in the supine position at night may experience reabsorption of water from the lower limbs and circulation, resulting in an increased volume of urine and therefore nocturia.27) Moreover, hypertension is a risk factor of obstructive sleep apnea, which results in a low oxygen level and an increased atrial natriuretic peptide level in the blood, thereby increasing the urine volume.29) This study confirmed the significant association between hypertension and nocturia in older adults, which is consistent with the results of the previous studies.
Several previous studies have reported the association between DM and nocturia.27,30) A prospective study has shown that DM in older adults can result in an increased urine volume at night, leading to nocturia and sleep disorder.31,32) Furthermore, several patients with DM have been reported to experience obstructive sleep apnea, which may have caused nocturia symptoms.33) The present study also confirmed the association between DM and nocturia in older adults.
The significance of this study lies in the confirmation of the prevalence of nocturia and identification of its associated factors in older adults, using a large population-based dataset. Furthermore, various subgroup analyses in this study confirmed that obesity was associated with nocturia regardless of sex, DM status, or hypertension status in older adults. However, this study also has several potential limitations. First, it was a cross-sectional study. To clarify the causality between obesity and nocturia, further prospective studies are necessary. Second, because of the lack of data, most participants in NHANES were excluded from this study. Therefore, selection bias may exist.
In conclusion, this study demonstrated that hypertension, DM, and obesity were significantly associated with the prevalence of nocturia in older people regardless of sex. In particular, obesity was associated with nocturia in every subgroup analysis.
Notes
The researchers claim no conflicts of interest.