Therapeutic Effects of Functional Electrical Stimulation on Physical Performance and Muscle Strength in Post-stroke Older Adults: A Review
Article information
Abstract
Stroke-related disabilities cause poor physical performance, especially among older adults, and can lead to sarcopenia. Functional electrical stimulation (FES) has been used to improve physical performance in individuals with neurological disorders and increase muscle mass and strength to counteract muscle atrophy. This review covers the principles, underlying mechanisms, and therapeutic effects of FES on physical performance and skeletal muscle function in post-stroke older adults. We found that FES restored weakened dorsiflexor and hip abductor strength during the swing and stance phases of gait, respectively, to help support weight-bearing and upright posture and facilitate static and dynamic balance in this population. FES may also be effective in improving muscle mass and strength to prevent muscle atrophy. However, previous studies on this topic in post-stroke older adults are scarce, and further studies are needed to confirm this supposition.
INTRODUCTION
Stroke, a neurological disorder attributed to focal injury of vascular origin to the central nervous system, is the third-leading cause of death worldwide and an important cause of disability in older adults.1-3) Approximately one-third of patients die owing to stroke, one-third experience secondary recurrent strokes, and most of the remaining patients live with mobility limitations.4) More than 80% of stroke survivors have gait impairment and often cannot walk independently to perform daily activities.5) Stroke-related disabilities are largely responsible for low physical performance, especially among older adults, and can also lead to sarcopenia because physical performance is a key aspect in sarcopenia development.6-8) Indeed, hemiparetic stroke can result in muscle abnormalities with denervation, disuse, inflammation, and remodeling of muscle tissues.9) This may be related to the disrupted synaptic transmission of motor neurons that innervate muscle fibers after stroke, which can lead to decreased number of motor units and changes in muscle structure.10) Hence, appropriate interventions are needed to prevent these changes. Identifying effective treatment strategies to improve gait disorders and the consequent loss of muscle mass after stroke is warranted.11) New modalities such as functional electrical stimulation (FES) are the most commonly used techniques to recover from foot drop.12)
FES dates back to the 1700s when Luigi Galvani conducted experiments on the leg muscles of frogs.13) In the 1800s, Guillaume-Benjamin Duchenne, who described the muscle disease “Duchenne muscular dystrophy,” developed a non-invasive technique to stimulate muscles using electric stimulus applied to the surface of the skin.13) Although FES has been utilized for several decades, it was not until the 1960s that its therapeutic uses and effects on skeletal muscles were more commonly reported.14) In 1961, Liberson et al.15) introduced a novel idea for the correction of gait disorder using electrical stimulation. Subsequent studies have reported that FES is an effective method to correct gait disorders in individuals post-stroke.16,17) Two meta-analyses also assessed the therapeutic effects of FES on muscle strength and physical performance in post-stroke patients; however, they did not consider muscle mass.18,19) Given recent findings on stroke-related sarcopenia, studies on the effect of FES on muscle mass, muscle strength, and physical performance should be systematically examined, especially in older adults. Therefore, this study reviewed the therapeutic effects of FES on physical performance and proposed hypotheses for its subsequent effects on muscle mass and muscle strength in post-stroke older adults.
FUNCTIONAL ELECTRICAL STIMULATION
Principles of FES
FES is the clinical application of electric current to a decentralized muscle. Neuromuscular electrical stimulation involves placing electrodes on a motor point and sending an electric current to produce muscle contraction.1) The technique can generate functional movements in individuals with paralysis caused by damage to the central nervous system. It entails the use of a low-energy electrical pulse and generates muscle contractions in a sequence to promote tasks such as walking or grasping.20) Specifically, FES electrically stimulates the dorsiflexor muscles (i.e., the tibialis anterior [TA]) of the foot, which facilitates ankle dorsiflexion during the swing phase of gait and allows for a more natural gait pattern.14)
FES has several advantages for gait training in patients with stroke. It can be used to enhance muscle strength and physical performance by increasing the range of motion and decreasing muscle weakness and spasticity.1,2,14) FES can also be used to relearn recruitment and timing of muscle activation in the paretic lower limb, which further helps in producing a normal gait. Individuals who experience stroke may have foot drop or weakness in the muscles lifting the foot during walking, which can further lead to falls or secondary health problems.21,22) If muscle contraction is appropriately timed and coordinated, gait performance can be facilitated in individuals with paralyzed lower extremities, such as patients who experience stroke.23) Thus, FES activation of muscles in the paralyzed limb is important to improve gait performance.
Types of Stimulation in FES
Electrical stimulation can be classified into invasive and non-invasive stimulation electrodes. Invasive stimulation electrodes are further separated into implanted and percutaneous electrodes, which differ in their placement duration and depth. Implanted electrodes are more suitable for longer-term use than percutaneous electrodes and are placed near the target nerve. Percutaneous electrodes are more suitable for short-term use than implanted electrodes and typically penetrate the skin by partially stimulating the targeted motor neurons.24) The typical current amplitude for both implanted and percutaneous electrodes is 25 mA. Invasive stimulation electrodes commonly require surgery; therefore, their placement and electrical intensity cannot be changed. In contrast, non-invasive stimulation electrodes self-adhere to the body surface. Unlike the fixed current amplitude of invasive stimulation electrodes, the typical current amplitude for non-invasive stimulation varies from 2 mA to 120 mA. Their placement on the skin also facilitates early intervention, which results in better recovery. Moreover, the electric intensity can be modified without surgery.25) However, targeting deep muscles is not feasible because stimulation of these muscles often requires greater intensity, which may result in stimulating untargeted muscles.
No studies have directly compared invasive and non-invasive electrical stimulation in post-stroke patients. However, previous studies have demonstrated the effectiveness of both methods in patients with spinal cord injury (SCI). Demchak et al.26) reported a greater cross-sectional area of the vastus lateralis muscle in the leg undergoing non-invasive electrical stimulation than that of the non-stimulated leg. Gad et al.27) also reported a higher increase in handgrip strength in the presence of non-invasive stimulation. The invasive approach also showed a restoring effect on walking and independent standing in individuals with SCI.28,29) Thus, both invasive and non-invasive electrical stimulation can help increase muscle cross-sectional area and muscle strength in patients with SCI. Furthermore, selecting an appropriate electrical stimulation method according to the patient’s condition is recommended.
Mechanisms of FES
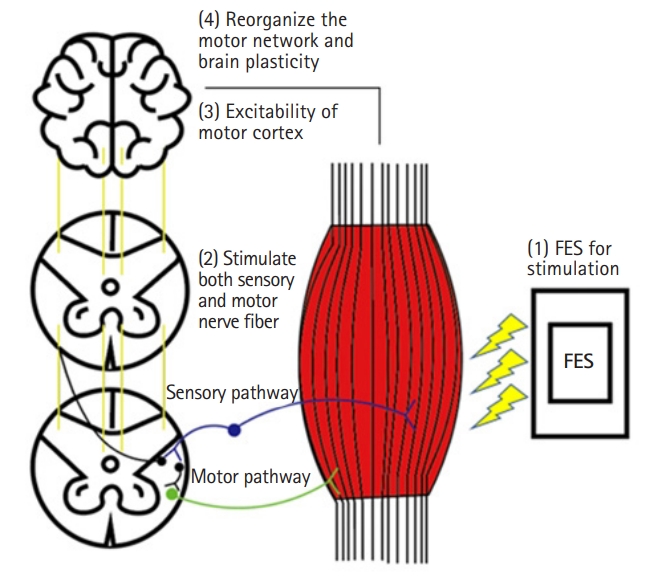
Although the clinical effects of FES on gait patterns have been reported,2,17) the mechanism is not yet clearly understood. “Central” and “peripheral” mechanisms have been proposed to describe the therapeutic effects of FES. The peripheral mechanism of FES involves improving the muscle strength, flexibility, range of motion, and muscle spasticity of the paralyzed limb. While these improvements in muscle fitness may appear to have lasting effects, none of the peripheral mechanisms can explain these lasting effects.30,31) Instead, the central mechanism has attracted increasing attention to account for these effects. The central mechanism of FES is as follows (Fig. 1). First, FES can stimulate both afferent sensory and motor nerve fibers. A previous preclinical showed that a change in afferent sensory signals was sufficient to reorganize the motor cortex.32) Prolonged peripheral stimulation can induce excitability of the human motor cortex and reorganize the motor networks of the corresponding muscles.33,34) The results of studies suggest that FES-triggered afferent feedback may facilitate persistent brain plasticity. Second, electrical stimulation can antidromically activate motor nerve fibers, which are then polarized when the antidromic impulses of the fibers reach the anterior horn cell. The combination of FES-induced antidromic impulse and voluntary movement promotes pre- and postsynaptic coupling and synaptic remodeling, which are necessary for changes in neural plasticity.30) Thus, these two hypotheses have been proposed as plausible mechanisms by which FES corrects and improves gait patterns.
Therapeutic Effects of FES on Gait in Post-stroke Patients
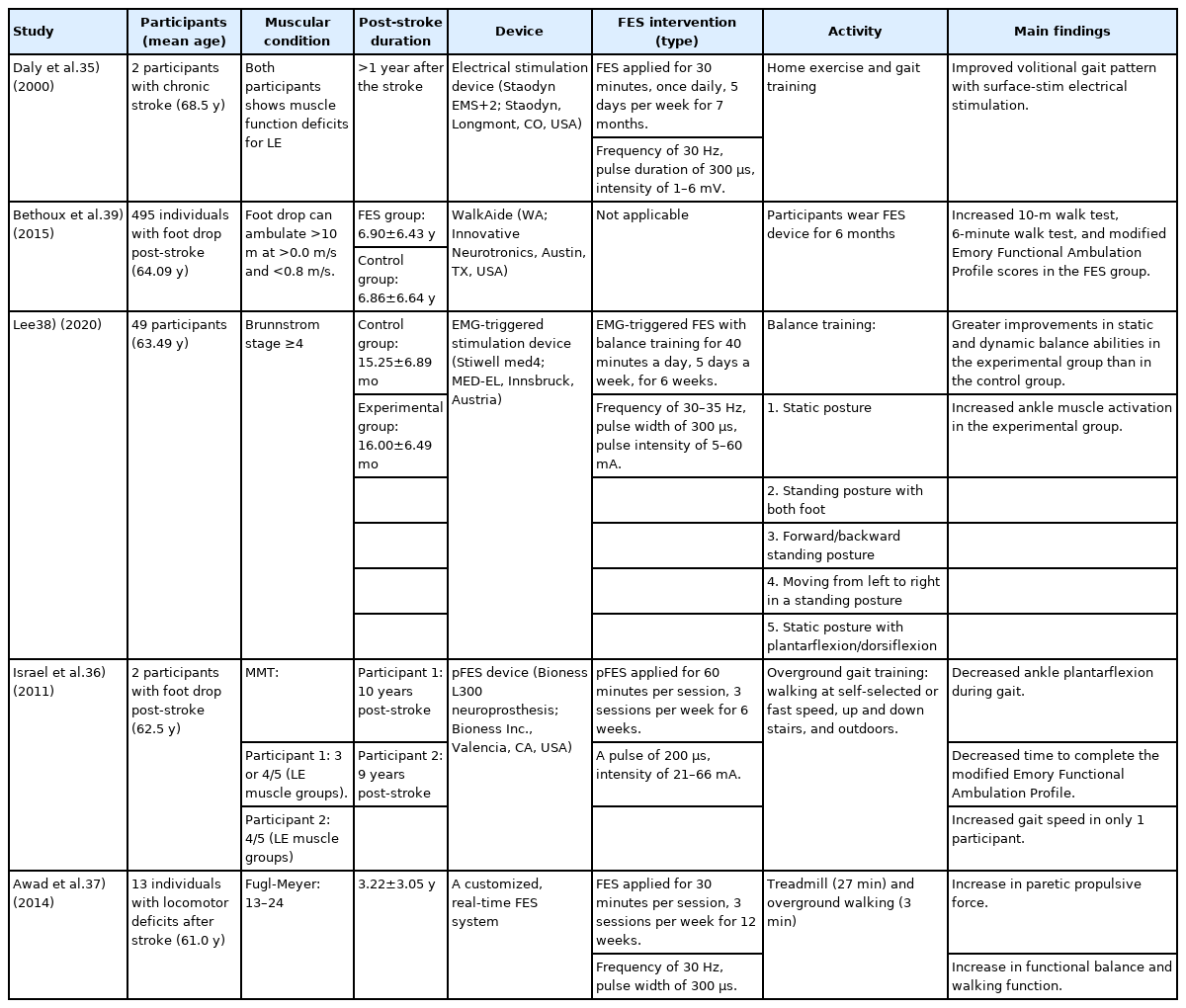
The benefits of FES on gait performance of patients with chronic stroke are summarized in Table 1. In their single-subject study, Daly et al.35) reported that gait training with functional neuromuscular stimulation improved the gait patterns in older adults with chronic stroke. Specifically, the use of a combined treatment resulted in significant improvements in volitional knee flexion function compared to conventional treatment alone. Israel et al.36) reported a case series of stroke patients, in which two participants showed improved functional ambulation and decreased ankle plantarflexion, demonstrating that overground gait training with FES can improve foot clearance during gait. In addition, a pilot study reported that patients who had experienced a stroke produced greater propulsive force via the combination of treadmill and overground walking at a maximal speed with FES, which was accompanied by improvements in functional balance and walking ability.37) As balance ability is an important goal of stroke rehabilitation, a recent study reported that the combination of balance training with FES was is acceptable and effective in improving static and dynamic balance.38) In a long-term follow-up randomized controlled trial over 12 months, FES showed similar effects to ankle-foot orthosis in all primary outcomes related to gait quality and function, suggesting that FES may be an appropriate alternative to orthosis for individuals with chronic stroke.39)
FES treatment of the dorsiflexors is effective in correcting foot drop to balance gait patterns after stroke but not in correcting asymmetric weight-shifting during gait.36) One reason for the asymmetric gait pattern is the lack of activation of the hip abductors, which work as pelvic stabilizers.40) A previous study reported that FES-triggered gait training of the gluteus medius (GM) and TA improved gait velocity, dynamic balance, and gait symmetry during walking, among patients aged <60 years.41) In that study, FES applied to the GM stabilized the pelvic muscles in the stance phase, while FES applied to the TA strengthened the ankle dorsiflexors in the swing phase, which improved functional gait performance in individuals with chronic hemiparetic stroke. These findings were consistent with those reported by Kim et al.,42) who demonstrated that FES applied to the GM in the stance phase and TA in the swing phase of gait improved gait performance in patients who had experienced a stroke. Specifically, the combined effect of GM activation with TA generates a more normal gait pattern than that generated by TA activation alone.42) Consequently, FES treatment of the hip abductors and dorsiflexors has the potential to improve gait symmetry and gait speed during walking. The recovery of stroke-induced gait disorders through FES treatment in middle-aged patients aged <60 years may have a positive effect on sarcopenia prevention in those aged >60 years.
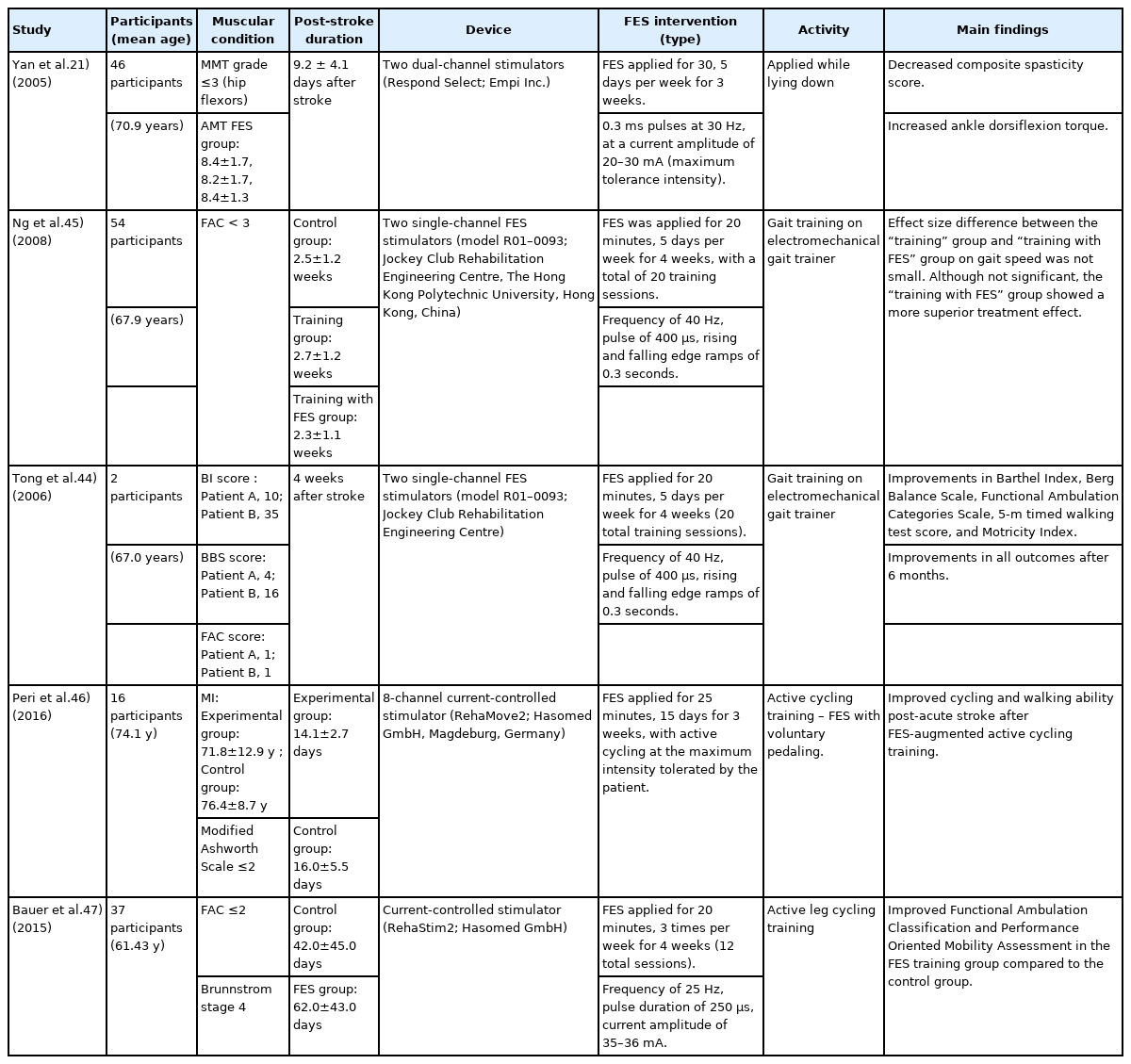
As in chronic stroke, FES also affects physical performance in patients with subacute stroke, as summarized in Table 2.43) Several studies have reported that FES can increase dorsiflexor strength in the swing phase of gait to prevent foot drop after stroke, which can further improve gait performance.21) Tong et al.44) reported that the therapeutic combined effect of FES and gait training was superior to gait training alone in individuals after acute stroke. They reported significant improvements in Barthel Index, Berg Balance Scale, Functional Ambulation Categories scale, 5-m timed walking test, and Motricity Index in the combination group compared to those in the training alone group after 4 weeks of treatment. Moreover, these improvements persisted even after 6 months. In one pilot study, the “gait training with FES” group had a larger effect size of gait speed than the “gait training only” group, indicating a superior treatment effect; however, no significant differences were observed.45) Another pilot study showed that active cycling training with FES may be effective in improving gait velocity in the subacute recovery period after stroke, although no group effect was found.46) The results of these pilot studies may have been more robust if the sample sizes were larger. A study comparing the effect of active interventions involving leg cycling with and without FES performed three times weekly for 20 minutes each session for 4 weeks showed improved walking and balance abilities in the FES group.47) Thus, the application of FES in the subacute phase of stroke may have a positive effect on physical performance. Several studies have demonstrated that FES helps patients with subacute and chronic stroke recover from low physical performance.
Therapeutic Effects of FES on Muscle Mass and Strength in Post-stroke Patients
However, few studies have demonstrated the effectiveness of FES in increasing muscle mass and strength in patients after stroke, especially in older adults. Several studies showed that FES significantly improved muscle strength in middle-aged patients with subacute and chronic stroke.48-50) Dorsiflexor muscle strength significantly increased by 56.6% in the “combination of FES and conventional rehabilitation” group and by 27.7% in the “conventional rehabilitation alone” group.49) These findings can be explained by the fact that FES reduces muscle spasticity through motor recovery, which further improves muscle strength. In addition, 18 patients with subacute and chronic stroke who participated in a 12-week conventional rehabilitation program combined with FES showed significantly improved dorsiflexor strength, measured by surface EMG signal.48) The combined effects of FES and rehabilitation also increased the maximal voluntary contractions of the dorsiflexors in middle-aged patients with stroke.50) The previous studies showing that FES increased muscle strength in middle-aged patients with stroke suggest the positive effects of FES on muscle mass, which is positively correlated with muscle strength. Muscle mass and strength may also be correlated in stroke survivors.51) Most studies were conducted among participants with a median age of 50 years, an age range that also encompasses older adults.48-50) Therefore, the same results may be observed in older adults, although studies with larger samples of older adults are required. Moreover, FES significantly restored muscle mass in denervated muscles of patients after SCI, although the patient age was relatively low.52) As stroke and SCI share common features, FES may also restore muscle mass in post-stroke patients.
In addition, FES may improve muscle mass by altering muscle-specific transcriptional mechanisms.53) During muscle contraction, muscle fibers produce and release myokines, which have local and systemic effects on the body.54) FES in older adults changes this myokine secretion, especially that of insulin-like growth factor-1 (IGF-1).55) A previous study showed that neuromuscular electrical stimulation induced increased expression of IGF-1 and its downstream pathways, a well-known major anabolic signal for skeletal muscle development, and decreased expression of MuRF-1 and Atrogin-1, which are muscle atrophy-related ubiquitin ligase genes.56) Considering these changes at the molecular level, FES may also be effective in counteracting muscle atrophy in older adults.
CONCLUSION
The findings of the current review suggest some benefits of FES in improving physical performance and muscle strength and increase the possibility of its subsequent positive effects on muscle mass in older adults with stroke. FES can also facilitate static and dynamic balance activities by strengthening weakened dorsiflexors in the swing phase and hip abductors in the stance phase to support weight-bearing and upright posture. Thus, FES, especially when combined with rehabilitation, can be used to optimize physical performance, including gait performance, and ameliorate the consequent loss of muscle mass and strength in older adults after stroke.
Notes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
This work was supported by a Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, and Ministry of Food and Drug Safety) (No. 1711138173, KMDF_PR_20200901_0101).
AUTHOR CONTRIBUTION
Conceptualization, CWW, MK; Data curation, CWW, HES; Funding acquisition, CWW, HL; Investigation, CWW, MK, YS, HES, JYJ, DL; Project administration, CWW; Supervision, CWW, MK; Writing–original draft, HES; Writing–review & editing, MK, DL, JYJ, YS, DHY, SK, JY, MKK, HL, CWW.