Development of Korean Frailty Index for Primary Care (KFI-PC) and Its Criterion Validity
Article information
Abstract
Background
The objective of this study was to develop and validate the Korean Frailty Index for Primary Care (KFI-PC) based on a comprehensive geriatric assessment.
Methods
We developed a 54-item KFI-PC comprising 10 standard domains: cognitive status including delirium or dementia; mood; communication including vision, hearing, and speech; mobility; balance; bowel function; bladder function; ability to carry out activities of daily living; nutrition; and social resources. To test its validity, we applied KFI-PC to participants of the Korean Frailty Aging and Cohort Study (KFACS). We analyzed 1,242 participants (mean age, 77.9±3.9 years; 47.2% men) from the KFACS who visited 10 study centers in 2018, after excluding 32 participants with missing data required to assess Fried’s physical frailty phenotype.
Results
The mean KFI-PC score was 0.17±0.08, ranging from 0.02 to 0.52. The median KFI-PC score was higher in women than in men, and there was a trend toward higher values in older age groups. The prevalence of frailty when applying a generally used frailty index cutoff point of >0.25 was 17.5% in the whole study sample. As a construct validation of KFI-PC, the area under the receiver operating characteristic curve for Fried’s physical frailty was 0.921, and the optimal cutoff value to predict frailty phenotype was 0.23. The KFI-PC score also correlated well with physical, cognitive, and psychological functions; nutritional status; disability in activities of daily living; and instrumental activities of daily living. The Cronbach’s alpha coefficient of the 54 total items was 0.737.
Conclusion
We developed KFI-PC with 53 deficits, including comprehensive geriatric assessment components, and demonstrated the acceptable construct validity and internal consistency of KFI-PC.
INTRODUCTION
Number of frail older people has been ever growing with the increase of global population aging. Frailty is defined as a status of vulnerability to identified stressors that exposes individuals to higher risks of negative health-related outcomes. The condition is usually caused by the interaction between progressive aging-related declines in multiple organ function and chronic diseases that often lead to a decreased level of functional reserve capacities.1)
Both phenotypic and deficit accumulation approaches are commonly used to define frailty. Representing the phenotypic approach, Fried’s frailty phenotype defines frailty as the presence of three or more of five frailty items; namely, slow walking speed, impaired grip strength, declining physical activity levels, exhaustion, and unintended weight loss.2) The other approach to defining frailty is through the use of a frailty index that sums health deficits. In this context, health deficits can be any physical or mental disability, symptom and sign, disease, laboratory finding, etc.3) Healthcare professionals have used comprehensive geriatric assessment (CGA) to develop a holistic overview of patients with complex needs, which is the essential step for the development of individualized, patient-centered care plans. CGA evaluates multiple aspects of older adults’ health, including cognition, emotion, motivation, health attitude, vision, hearing, speech, sleep, pain, strength, balance, mobility, activities of daily living, social engagement, medication, control of life, etc. In primary care settings, frailty indices can be developed based on CGA.
A CGA-based frailty index (FI-CGA) was first developed using clinical examination data from the Canadian Study of Health and Aging.4,5) The standardized CGA used to constitute the frailty index comprises assessments in 10 standard domains: (1) cognitive status including delirium or dementia; (2) mood and motivation; (3) communication including vision, hearing, and speech; (4) mobility; (5) balance; (6) bowel function; (7) bladder function; (8) instrumental activities of daily livings (IADLs) and activities of daily living (ADLs); (9) nutrition; and (10) social resources.4) Based on this principle, Theou et al.3) constructed FI-CGA containing 56 variables chosen from among a CGA adapted for use within the primary care setting.
The authors demonstrated that FI-CGA was feasible to assess frailty in primary care for a multidisciplinary primary care program for frailty. Additionally, FI-CGA was useful for the care of frail older persons in primary care as any specific problems out of 10 domains can be identified and managed effectively. Following these principles and the example of FI-CGA in Canada, we developed a Korean Frailty Index for Primary Care (KFI-PC) and investigated its validity and reliability.
MATERIALS AND METHODS
Development of KFI-PC
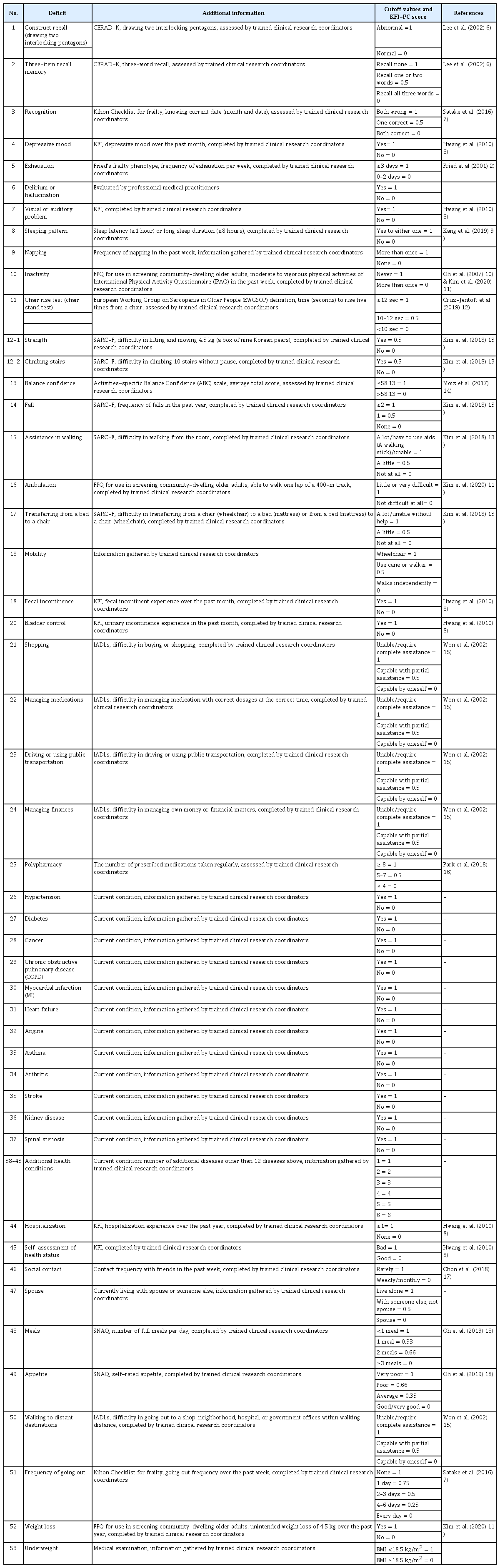
The deficits included in KFI-PC, along with their cutoff values, scoring measures, and related references, are described in Table 1.2,6-18) The Korean version of the KFI-PC is provided in Supplementary Table S1. We adopted questionnaires or assessments validated in Korea for items of KFI-PC while referring to FI-CGA and the validated Korean frailty indices. We replaced or excluded items that were not appropriate for use in busy primary care settings in Korea; for example, “low mood” in FI-CGA was excluded because it is duplicated with the evaluation of “depression”. We also excluded “motivation”, “health attitude”, and “control of life events” because they were not appropriate for Korean older adults. We excluded the timed up and go test because it requires a 3-m length of space to perform; it was replaced by a chair stand test (rising from a chair five times).19) We also excluded IADLs of cooking and cleaning as those activities are not appropriate to assess older Korean men. We replaced these IADLs with “walking to distant destinations”. FI-CGA also includes the Montreal Cognitive Assessment; however, as it takes more than 20 minutes to complete, we replaced it with the Mini-Cog test. The Mini-Cog test combines two simple cognitive tasks (a three-item word memory and clock drawing) with a scoring algorithm.20) It can be completed in 2–4 minutes and has shown high diagnostic accuracy for dementia (sensitivity 76%, specificity 99%). We included factors related to hospital admission within 1 year and self-assessment of health as they are included in the Korean frailty index.8) Contact frequency with friends,17) living with family (a spouse), and frequency of going out of the home7) were included as known social risk factors for frailty. Finally, we included data regarding appetite and number of full meals eaten per day from the Short Nutritional Assessment Questionnaire (SNAQ) as nutritional assessment.18) Regarding comorbidities, FI-CGA allowed a maximum of 18 current conditions. The comorbidities included hypertension, diabetes, cancer, chronic obstructive pulmonary disease, myocardial infarction, heart failure, angina, asthma, arthritis, stroke, and kidney disease as they are embedded in the Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight (FRAIL) questionnaire.21) Spinal stenosis was included as the 12th disease to be questioned.22) If the subjects had other diseases, each additional condition was recorded up to 18 diseases. We selected these items through article review and the consensus of three experts and authors (CWW, MK, and YL).
KFI-PC Scoring
In this study, similar to the FI-CGA scoring strategy, each deficit item was scored up to 1 point except for strength (item# 12-1) and climbing stairs (item# 12-2), which represented muscle strength of the upper and lower extremities, respectively. As suggested by Rockwood and Searle, each deficit variable was dichotomized or polychotomized and mapped to the interval 0–1 (e.g., for self-rating of health, “Excellent” was coded as 0, “very good” as 0.25, “good” as 0.5, “fair” as 0.75 and “poor” as 1) to represent the deficit frequency or severity.23) Although KFI-PC includes a total of 54 items, the maximum deficit score is 53 as the questions on strength (item# 12-1) and climbing stairs (item# 12-2) had maximum scores of 0.5. The final scoring method was decided based on the consensus of the three experts. In general, missing variables can be imputed or removed from the denominator.24) This study followed the latter approach of scoring KFI-PC. The KFI-PC score of each participant was calculated by dividing the number of deficits by the number of total variables that were recorded for that patient. For example, we divided the total score of deficits by 53 for patients with recorded data for all variables. If a patient was missing data on two variables, then the number of deficits for this patient was divided by 51. If data on one of the strength or climbing question was missing, the total KFI-PC score was calculated by dividing by 52.5. In this way, the KFI-PC score is continuous (0 to 1), with higher scores indicating an increased likelihood of frailty.
Study Sample and Study Design
To establish the feasibility and preliminary validity analysis of KFI-PC, we used cross-sectional data from the Korean Frailty Aging and Cohort Study (KFACS). KFACS is a multicenter longitudinal study whose participants were recruited from among community-dwelling residents in urban and rural areas nationwide in 10 study centers across different regions.25) Each center recruited participants using quota sampling stratified by age and sex at local senior welfare centers, community health centers, apartments, housing complexes, and outpatient clinics. We used quota sampling based on age (70–74, 75–79, and 80–84 years with a ratio of 6:5:4, respectively) and sex (male, female) with an aim of recruiting 1,500 men and 1,500 women. The inclusion criteria were age 70–84 years, living independently at home, having no plans to move out in the next 2 years, and no problems with communication due to serious cognitive impairment. The first wave of baseline data collection started in 2016–2017; of 3,014 participants who underwent baseline survey, 1,559 (51.7%) and 1,455 (48.3%) were enrolled in the study in 2016 and 2017, respectively. The follow-up rate in 2018 (baseline survey in 2016) was 92.5%, with 88.4% visiting the clinical sites, 11% completing telephone interviews, and approximately 0.5% involving home visits. This study included its sample from the second wave of a 2016 baseline survey, from among the 1,274 participants who visited the 10 study centers in 2018 as SNAQ was first included in the second wave in 2018. KFI-PC was assessed in on-site clinical examinations. The final analysis included 1,242 participants, after excluding 32 participants who did not have the data required to assess the Fried’s physical frailty phenotype.
Ethics
The KFACS protocol was approved by the Institutional Review Board (IRB) of the Clinical Research Ethics Committee of Kyung Hee University Hospital, Seoul, Korea, and all subjects provided written informed consent (No. 2015-12-103). The present study was exempt from the requirement for IRB approval by the Clinical Research Ethics Committee of Kyung Hee University Hospital (No. 2020-04-033).
Assessment of Fried’s Physical Frailty Phenotypes
This study defined physical frailty using a modified operational definition of Fried’s physical frailty phenotypes from the Cardiovascular Health Study (CHS).2) The five different components of frailty indicators were (1) weight loss: answering “yes” to “In the last year, have you lost more than 4.5 kg unintentionally?”; (2) weakness: maximal grip strength in the lowest 20% of the weighted KFACS population distribution, adjusted for sex and body mass index; (3) slowness: 4-m usual gait speed in the lowest 20% of the weighted KFACS population distribution, adjusted for sex and height; (4) exhaustion: answering “yes” to either one of the following statements from the Center for Epidemiological Studies-Depression scale “I felt that everything I did was an effort” or “I could not get going” for three or more days per week; and (5) low physical activity: kilocalorie per week (kcal/week) expenditures were calculated for each activity using its metabolic equivalent score using the International Physical Activity Questionnaire, with low physical activity defined as <494.65 kcal for men and <283.50 kcal for women, which was the lowest value for 20% of the sex-specific total energy consumed from a general Korea population-based survey of older adults.26) Although the Physical Activity Scale for the Elderly (PASE) is one of the most commonly used methods, the Korean version takes up to 10 minutes to administer. A Korean study found moderate to high agreement between the CHS frailty phenotype definitions based on the K-PASE or International Physical Activity Questionnaire short form.27) In this context, subjects with three or more components were considered to have physical frailty.
Statistical Analysis
Data are presented as mean±standard deviation or as numbers (percentages). Continuous variables were compared using independent t-tests, and categorical variables were compared using chi-square or Fisher exact tests. We used Shapiro–Wilks tests to assess normality and Mann–Whitney U tests and Kruskal–Wallis tests to assess KFI-PC scores with respect to sex and age groups. Significant differences in KFI-PC scores between age groups were assessed using non-parametric post-hoc tests with Mann–Whitney U tests (p<0.016). The internal consistency of the 54 items was assessed based on Cronbach’s alpha coefficients. For construct validation of KFI-PC-index, we used Spearman rank correlation coefficients (rs) to explore the relationships between KFI-PC score and outcomes. Receiver operating characteristic (ROC) analysis was performed to explore the cutoff values of the KFI-PC score and to verify the criterion validity for frailty according to Fried’s physical frailty phenotype. The optimal cutoff values with the greatest sum of sensitivity and specificity for correctly identifying frail individuals were determined using Youden’s index. The statistical analyses were performed using Stata (version 14.0; Stata Corp., College Station, TX, USA) and IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA). Two-tailed p<0.05 indicated statistical significance in this study.
RESULTS
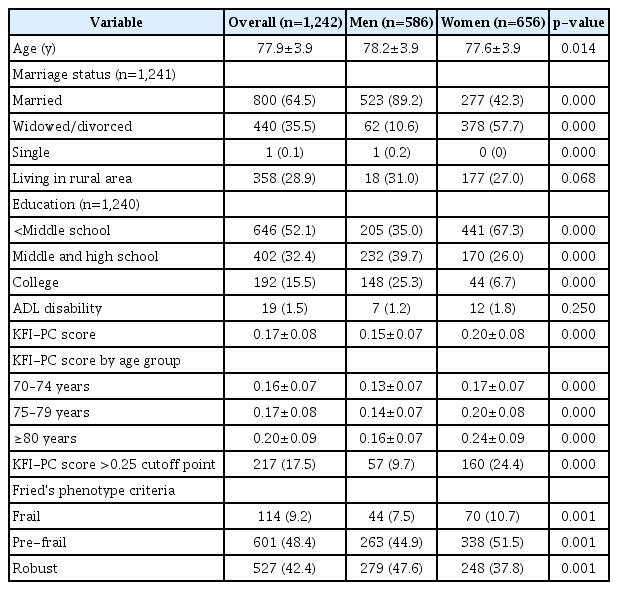
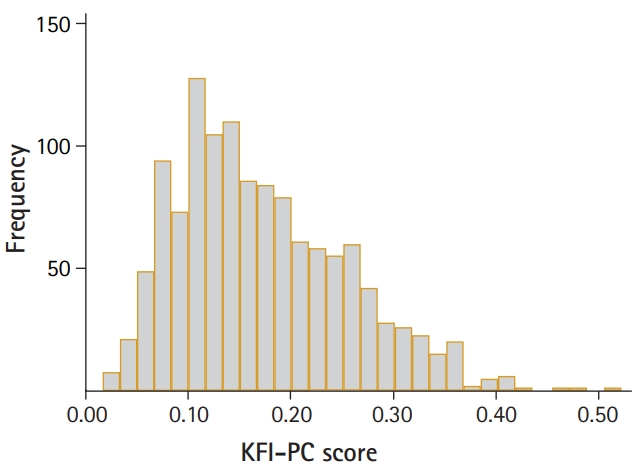
Table 2 shows the characteristics of the study participants. Overall, the mean age was 77.9 and 28.9% of participants were living in rural areas. As the KFACS cohort study included participants who could visit 10 centers, ADL disability in any of five basic activities of daily living (i.e., dressing, bathing, toileting, transferring, and feeding) was rare (1.5%). Furthermore, the average overall KFI-PC score was 0.17. The KFI-PC score was higher in women and older groups in both sexes. The median and quartile KFI-PC scores for men and women and for age groups are shown in Supplementary Table S2. The KFI-PC scores showed a right-skewed distribution ranging from 0.02 to 0.52 (Fig. 1). Participants with KFI-PC score over 0.25, usually recognized the cutoff of frailty, represented 17.5% of the total population; however, the frailty prevalence by Fried’s phenotype criteria was 9.2%. The KFI-PC score increased with age levels and the pattern was more exaggerated in women (Fig. 2). The deficit scores and missing data for each item of KFI-PC are presented in Table 3. The highest saturated deficit score was 60.2% with the current condition of hypertension. The highest rate of missing was 1.4% for the sleeping pattern item. The Cronbach’s alpha coefficient of the 54 items total was 0.737, within the acceptable range (0.7 or above) for internal consistency (reliability).
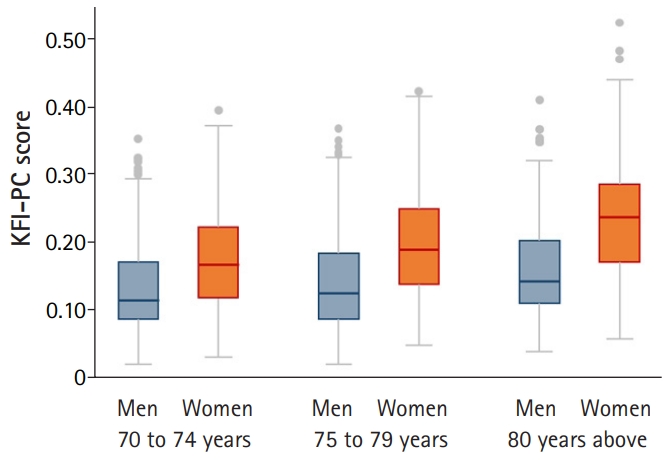
Boxplot of the Korean Frailty Index for Primary Care (KFI-PC) scores for men and women and for three age groups. The median (horizontal line) is shown within each box. The KFI-PC score differed significantly between men and women in all age groups (p<0.001) and between the three age groups in men and women (p<0.01) except for 70–74 years vs. 75–79 years in men (p=0.144).
Construct Validity of KFI-PC
To assess the construct validity (convergent validity) of KFI-PC, we compared it to Fried’s physical frailty (Fig. 3, Table 4). ROC analysis performed to confirm the criterion-related validity of KFI-PC for Fried’s physical frailty showed an area under the curve of 0.921 (95% confidence interval, 0.910–0.940). The ROC analysis revealed an optimal cutoff value, statistically defined as the best compromise between sensitivity and specificity, of 0.23 (sensitivity=89%, specificity=81%). The KFI-PC score showed correlations with physical, cognitive, and psychological functions, as well as nutritional status, disability in ADLs, and IADLs irrespective of age and sex (Table 4).
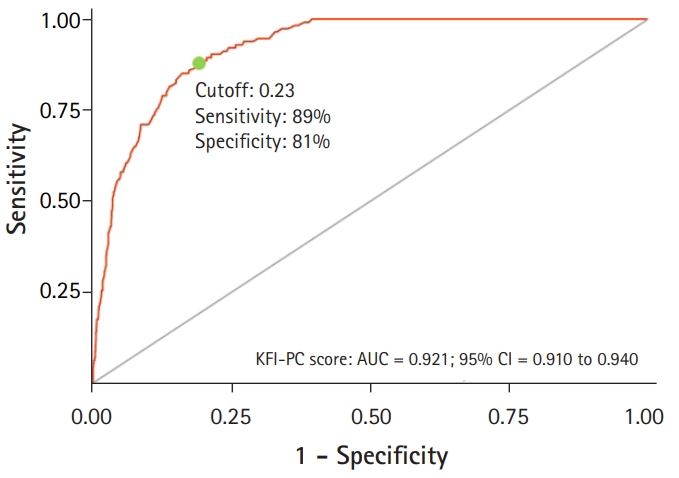
Receiver operating characteristic (ROC) curve of the Korean Frailty Index for Primary Care (KFI-PC) score according to Fried's phenotype criteria. AUC, area under the ROC curve; CI, confidence interval.
DISCUSSION
We developed a KFI-PC containing 54 items with a maximum deficit score of 53 and demonstrated its acceptable internal consistency and construct validity. Broadly speaking, KFI-PC is a comprehensive assessment that covers health-related areas related to cognitive, mental, physical, social, and nutritional factors, as well as ADLs and medical illness.
Generally, frailty indices should contain at least 30 items and cover a range of health indicators including chronic conditions, physical/cognitive limitations, and general health. Another characteristic of frailty index is that each deficit should be health-related and increase with age.24) Previous studies used 30–70 deficits to construct frailty indices. However, Searle et al.23) recommended that frailty indices should include at least 30–40 total deficits. Another criterion is that the deficit should not saturate too early, i.e., it should not be present in all or most people. A reasonable criterion for saturation appears to be about 80% or less as any deficits present in more than 80% of people do not make a significant difference in grading frailty.28) KFI-PC satisfied all these requirements. Moreover, it covers a range of not only chronic conditions, physical/cognitive limitations, and general health but also the factors related to social and psychological health.
In this study, the ROC analysis demonstrated an optimal KFI-PC cutoff value of 0.23, consistent with the consensus cutoff point for frailty of 0.25 for the frailty index used to define frailty in other studies.29) The original paper suggested a frailty cutoff of 0.25 based on a physical frailty index containing 70 deficits and data from participants aged 70 years and older in the Canadian Study of Health and Aging. However, another paper proposed a frailty cutoff of 0.21.30) A study analyzing Canadian Health Survey data from participants aged 65 years and over reported that the risk of hospital-related events increased at a value of 0.21. The cutoff is the lowest point for predicting outcomes; it may be sensitive but not specific and, therefore, not the optimal threshold.
Regarding participants with missing variables, studies commonly exclude any item with more than 5% of missing data31) and any participant with at least one missing item from more than 20% of the items.30) In this study, 40 of 53 (75.5%) items had complete data. Of the 13 items with missing data, 10 items were missing only 1 or 2 value; the other three items had 3, 6, and 18 missing values. Thus, missing variables were not an issue in this study. KFI-PC is easily evaluated in primary care, as it is mainly made of self-responding questionnaires, with only the Mini-Cog and chair rise tests requiring healthcare provider evaluations. The Mini-Cog test can be completed in 2–4 minutes. The chair rise test takes approximately 1–2 minutes to administer after a simple demonstration. The chair rise test can be used as an alternative for gait speed or handgrip strength. It is particularly valuable and applicable to studies that do not or cannot include gait testing due to a lack of space or instrument to measure handgrip strength.
The KFI-PC score increased with age levels, a pattern that was more pronounced in women. Previous studies reported that deficits consistently accumulate exponentially with age at an average relative rate of approximately 3% per year on a log scale and that in general, at any given age, women on an average have more deficits than do men.32) The reason for the sex difference may be mainly because of a higher incidence of comorbidities in women than in men, in addition to social, behavioral, and biological differences between men and women.33)
We observed a frailty prevalence of 9.2% based on Fried’s phenotype criteria and 17.5% based on KFI-PC, with a cutoff of 0.25. This result is compatible with that of previous reports of a 10% higher frailty prevalence using the frailty index compared with that using the phenotype criteria.34) The frailty index is associated with adverse health outcomes even among people categorized as non-frail by frailty phenotype.34) This finding suggests that the frailty index is a more sensitive measure for determining frailty owing to its ability to detect this condition at even the early stage of a frailty trajectory.34) Furthermore, the continuous nature of the frailty index allows it to trace slight changes in frailty to intervene before an individual reaches a definite frail phenotype.33) The prevalence of ADL disability in this study was only 1.5%. As the participants of the KFACS are comparatively healthy older adults who can visit the centers, the percentage of ADL disability may be lower than other home visit surveys. However, KFI-PC was developed for use in outpatient primary care and those patients must be ambulatory to visit clinics. In comparison, the reported prevalence of ADL disability was 2.6% in four outpatient clinics and two welfare centers.35)
In conclusion, we developed KFI-PC containing 53 deficits including comprehensive geriatric assessment components. KFI-PC comprises mainly self-administered questionnaires; only the Mini-Cog and chair rise tests are assessed by medical personnel and require limited time to perform. We demonstrated the construct validity and internal consistency (reliability) of KFI-PC. KFI-PC is easily assessed, was not considered a burden on the medical personnel who practice in primary care, and was well validated. Further studies are needed to determine whether KFI-PC is a good indicator for the prevention of adverse health outcomes and if it is feasible in real-world primary care settings.
Notes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
This research was supported by a grant from the Korea Health Technology R&D Project through the Korean Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (No. HI15C3153).
AUTHOR CONTRIBUTIONS
Conceptualization, CWW; Data curation, CWW, SL, MK; Funding acquisition, CWW; Investigation, CWW, SL, YL, MK; Methodology, CWW, SL, YL, MK; Project administration, CWW; Supervision, CWW; Writing-original draft, CWW, MK; Writing-review & editing, CWW, SL, YL, MK.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4235/agmr.20.0021
KFI-PC in Korean version
Median and quartiles (Q1, Q3) of the Korean Frailty Index for Primary Care scores for men and women and for three age groups