Deep Vein Thrombosis in a Patient with Negative Age-Adjusted D-Dimer Level
Article information
Abstract
D-dimer level, along with a clinical probability tool that uses the Wells score, is commonly used to exclude deep vein thrombosis (DVT). Age-adjusted D-dimer values are routinely used in clinical practice to increase the negative predictive value and avoid unnecessary Doppler ultrasound imaging. We describe a patient with a low pre-test probability of DVT upon admission and a negative D-dimer level based on age-adjusted values who was later diagnosed with DVT. Our experiences with this case highlight that the geriatric population is unique and, at times, frail.
INTRODUCTION
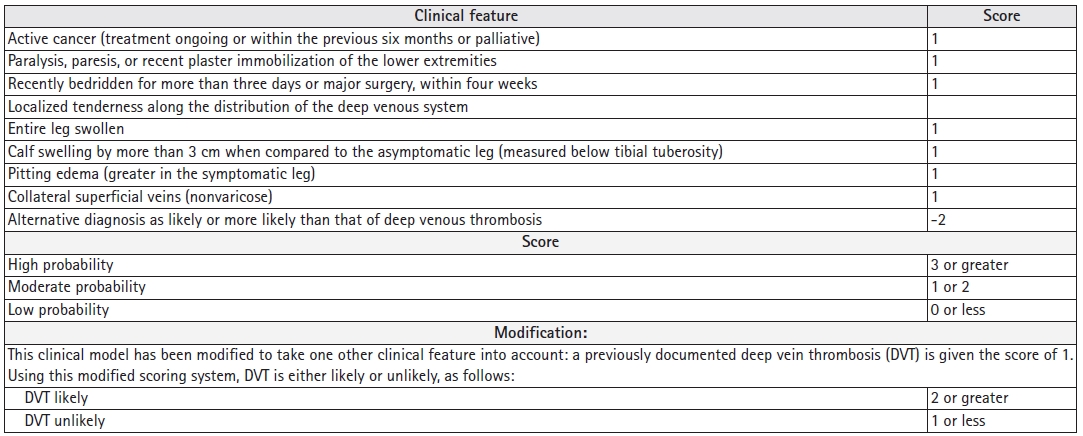
Deep vein thrombosis (DVT) is a common diagnosis encountered in the hospital. The Centers for Disease Control and Prevention (CDC) reported an estimated 900,000 cases of venous thromboembolism each year in the United States with 60,000–100,000 deaths. Among these cases of mortality, 25% present with sudden death as the first symptom of pulmonary embolism (PE), for which DVT is a risk factor.1) PE is a common clinical problem in geriatric populations with immobility secondary to various reasons.2) Most clinicians follow a diagnostic algorithm that starts with the determination of the clinical pre-test probability (PTP) based on D-dimer levels. The Wells score and modified Wells score are commonly used and widely studied to determine PTP,3,4) as summarized in Fig. 1. In patients with low PTP in the Wells test or unlikely results in the Modified Wells, the use of D-dimer assessment to exclude DVT is recommended, with a conventional D-dimer cutoff value of <500 ng/mL.5) However, D-dimer levels increase with age, hampering the specificity of D-dimer-based assessments in older patients. Using a higher D-dimer cutoff in older patients improves the diagnostic utility and specificity. One meta-analysis of 13 cohorts (12,497 patients) comparing the specificity of conventional D-dimer cutoff values (<500 ng/mL) to age-adjusted values—defined as age (year) × 10 ng/mL for patients aged >50 years—showed that the specificity of the conventional cut-off value decreased with increasing age, from 57.6% (95% confidence interval [CI], 51.4%–63.6%) in patients aged 51–60 years to 39.4% (95% CI, 33.5%–45.6%), 24.5% (95% CI, 20.0%–29.7%), and 14.7% (95% CI, 11.3%–18.6%) in those aged 61–70 years, 71–80 years, and >80 years, respectively. Age-adjusted cut-off values revealed higher specificities for all age categories—62.3% (95% CI, 56.2%–68.0%), 49.5% (95% CI, 43.2%–55.8%), 44.2% (95% CI, 38.0%–50.5%), and 35.2% (95% CI, 29.4%–41.5%), respectively. The sensitivities of the age-adjusted cut-offs remained >97% in all age categories.6) If DVT is not ruled out based on PTP and D-dimer levels, compression ultrasonography (CUS) with Doppler of the whole leg is the diagnostic test of choice in patients with suspected DVT. Using the ultrasound probe pressure, the presence of a thrombus is diagnosed by demonstrating the noncompressibility of the imaged vein. The veins that can be assessed for compressibility are the proximal (e.g., common femoral, femoral, and popliteal) and distal (e.g., peroneal, posterior, anterior tibial, and muscular) veins. The risk of embolization is higher in proximal than in distal DVT, and >90% of acute PE arises from the proximal veins.7) The Wells score has been validated in outpatient and emergency department settings; however, a study evaluating the Wells score for inpatients showed that it performed only slightly better than chance for the discrimination of DVT risk in hospitalized patients. The Wells score showed a higher failure rate and lower efficiency in the inpatient setting compared to reports in the outpatient literature. Therefore, risk stratification based on the Wells score is not sufficient to rule out DVT or to influence management decisions in inpatient setting.8) This brings up the argument for hospitalists to decide how to use D-dimer measures and how to interpret PTP and D-dimer levels without anchoring bias from emergency departments or admitting providers.
CASE REPORT
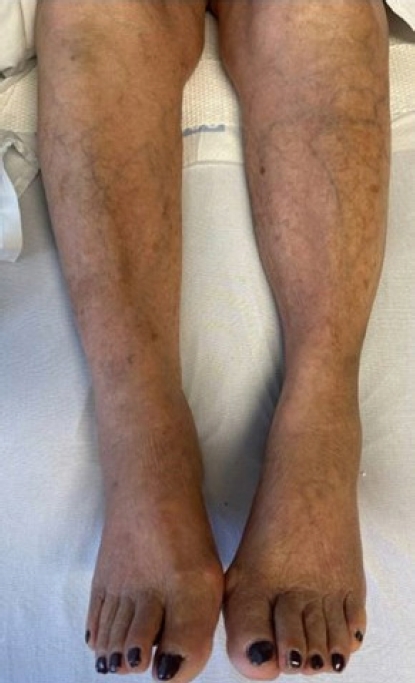
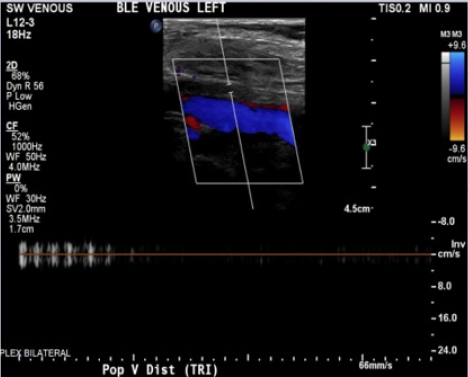
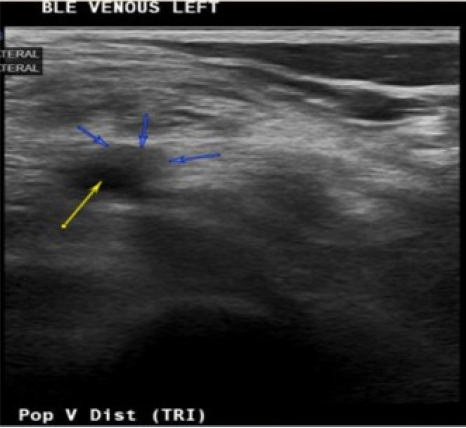
An 85-year-old Spanish-speaking woman with a medical history of essential hypertension and diastolic congestive heart failure (CHF) and a questionable history of remote DVT after giving birth to her son presented to the emergency department (ED) for further evaluation of hypertension and bilateral lower extremity edema that had persisted for approximately 2 weeks. The review of the electronic medical records indicated that the patient had been admitted to the hospital 3 months prior with similar complaints. An echocardiogram performed at that time revealed mild diastolic dysfunction and otherwise normal findings. The patient was discharged on routine medications, including amlodipine (5 mg daily), losartan potassium (50 mg daily), and furosemide (20 mg daily), and counseled on reducing salt intake as dietary noncompliance was the reported main reason for the swelling. Since her last admission, the patient had been started on losartan/hydrochlorothiazide (100 mg/12.5 mg) daily when she visited Mexico, and the furosemide was discontinued. In the ED, the patient’s workup was significant for hyponatremia, with a sodium level of 127 mEq/L and normal renal and liver function profiles. The patient’s result was negative for troponin, and the brain natriuretic peptide (BNP) concentration was 84 pg/mL. The patient’s vital signs included the following: blood pressure 164/84 mmHg; pulse 62 beats/minute; temperature 98.1°F; respiratory rate 16/minute; and oxygen saturation (SpO2) 98% on room air. Chest radiography was negative for the acute process, and the electrocardiogram findings were normal. The physical examination was unremarkable except for 2+ pitting bilateral edema. In the ED, the patient was administered an intravenous dose of furosemide and was admitted for hyponatremia and lower extremity swelling. The patient’s sodium level normalized with fluid restriction, and hydrochlorothiazide was discontinued over the next 2 days. Losartan was continued and enoxaparin (40 mg) was initiated for DVT prophylaxis. One dose of furosemide reduced the swelling. Upon admission, the patient’s lower extremity swelling was thought to be secondary to the history of diastolic CHF, dietary noncompliance, and the contribution of amlodipine. The patient’s D-dimer level was 0.72 μg/mL fibrinogen equivalent units (FEU) (reference range: 0.00–0.50 μg/mL FEU), which was a negative finding based on the age-adjusted cut-off. The next day, the patient mentioned that along with bilateral lower extremity edema, mild left calf pain was also present, which the patient was not able to further characterize and felt was mild and insignificant. A detailed history at that point indicated that the patient had come to the United States from Mexico approximately 2 months before. The patient denied having experienced any recent trauma, surgery, or hospitalization and also denied a family history of clotting disorders. Regarding the remote history of DVT, the patient mentioned a clot in the left leg that had required surgery after giving birth; however, the patient did not remember ever being on blood thinners and could not provide any other details of the surgery. On examination, the patient had bilateral lower extremity swelling, measuring 15 cm in the right leg and 16 cm in the left leg. Tenderness in the left leg was also present, which the patient denied upon admission to the ED. More swelling was observed in the right ankle than in the left ankle; otherwise, the swelling was bilaterally similar, as shown in Fig. 2. Although the patient’s D-dimer level was 0.72 μg/mL FEU, given the clinical picture without evidence of true heart failure to explain the lower extremity swelling and no other reason to explain the pain, we ordered CUS, which revealed occlusive DVT inferiorly at the trifurcation of the left popliteal vein, as shown in Figs. 3 and 4. The patient was initiated on a heparin drip and switched to apixaban upon discharge. On admission and during the hospital stay, the patient denied any shortness of breath, chest pain, cough, or blood-tinged sputum. Given that the patient had no symptoms of PE such as dyspnea, chest pain, or cough; normal chest X-ray and negative troponin findings; and normal BNP levels, in addition to CUS showing distal DVT, which is a less common cause of PE, we decided not to perform chest computed tomography angiography (CTA) because the likelihood of PE was low, the cost of CTA was prohibitive for the patient, and the findings would not change management of this case. Informed consent was obtained.
DISCUSSION
Based on our patient’s presentation in the ED and upon admission, the working diagnosis of the lower extremity swelling was multifactorial, including diastolic CHF, dietary noncompliance, and amlodipine use. The patient had no history of active cancer, paralysis, paresis, or recent immobilization and had not recently been bedridden for >3 days or undergone major surgery within 4 weeks. The patient had traveled from Mexico to the United States 2 months prior. The bilateral leg swelling showed a difference of <3 cm. The patient had superficial collateral veins bilaterally. However, an alternative diagnosis as likely as or more likely than that of DVT was possible, and it negated two points on both Wells and Modified Wells scores; therefore, PTP showed a low probability or was unlikely. Therefore, her D-dimer level was negative based on age-adjusted limits, and in clinical practice, CUS was not needed. However, our experiences with this case highlight the fact that even though the patient’s left calf pain was nonspecific, reevaluation of the odds of DVT at follow-up is important. Moreover, in this kind of presentation, with multiple possible explanations for the symptoms, a negative D-dimer finding might prevent us from considering the possibility of DVT due to an anchoring bias. Amlodipine is associated with pedal and lower extremity edema, which is a common dose-dependent side effect if taken for >4 weeks.9) The patient’s history of uncontrolled hypertension, diastolic heart failure, and dietary noncompliance were all likely reasons for the presentation. Therefore, even after adding isolated left leg pain, it can be argued that the modified Wells score changed. If multifactorial reasons cannot explain symptoms such as the left leg pain in the present patient, D-dimer levels should not be considered in the diagnosis, and on a follow-up visit, patients should undergo CUS testing to rule out DVT. This case emphasizes the importance of not having an anchoring bias and keeping an open mind when evaluating a patient when a new complaint arises during independent history-taking or when patients mention small non-significant complaints during their hospital stay. Silveira et al. reported that the usefulness of the Wells scoring system has not been validated; additionally, more data are needed to determine when to stop using the Wells scoring system after a patient is admitted.
In conclusion, DVT is associated with local and life-threatening complications, including death from PE. Proximal DVTs are major, life-threatening complications. The subjective differences among calculators must be considered when determining the PTP for DVT. However, the use of the Wells score in inpatient settings should be questioned. This case also highlights that anchoring bias can occur if we do not change our interpretation of D-dimer after more information in terms of history or diagnosis becomes clearer on the days following admission, as usually upon admission, patients are managed based on a working diagnosis.
Notes
CONFLICT OF INTEREST
Dr. Satnam Kaur Kulkarni and Shelby Reesing for providing Ultrasound Images. The researcher claims no conflicts of interest.
FUNDING
None.
AUTHOR CONTRIBUTIONS
The author performed the design and implementation of the proposed method and read and approved the final manuscript.