 |
 |
- Search
| Ann Geriatr Med Res > Volume 27(2); 2023 > Article |
|
Abstract
Background
Matrix metalloproteinases (MMPs) play an important role in bone resorption and are regulated by tissue inhibitors of metalloproteinases (TIMPs). We investigated the use of MMP2/TIMP2 and MMP9/TIMP1 ratios as biomarkers of bone resorption in geriatric osteoporosis and evaluated the relationship between osteoporosis and geriatric syndromes.
Methods
This analytical cross-sectional study involved 87 patients (41 with osteoporosis) treated at the geriatric outpatient clinic of a university hospital. The demographic characteristics, comprehensive geriatric assessment scores, laboratory findings, and bone mineral density of the patients were recorded. Serum MMP9, TIMP1, MMP2, and TIMP2 levels were analyzed by enzyme-linked immunosorbent assay (ELISA).
Results
We enrolled 41 and 46 patients with and without osteoporosis, respectively. The groups showed no significant differences in MMP2/TIMP2 and MMP9/TIMP1 ratios (p=0.569 and p=0125, respectively). While the basic activities of daily life (BADL) scores in the osteoporosis group were higher than those in the group without osteoporosis, the instrumental activities of daily life (IADL) scores were significantly lower (p=0.001 and p=0.007, respectively). No significant differences were observed in Mini-Nutritional Assessment, Mini-Mental State Examination, and Geriatric Depression Scale scores (p=0.598, p=0.898, and p=0.287, respectively).
Conclusion
This is the first study to examine the relationship between osteoporosis and several geriatric syndromes, as well as the relationship between osteoporosis and serum MMP, TIMP values, and MMP/TIMP ratios in geriatric patients. Our results showed that osteoporosis causes dependency in both BADLs and IADLs, and that the MMP2/TIMP2 and MMP9/TIMP1 ratios provided no additional benefit in demonstrating bone resorption in geriatric osteoporosis.
Osteoporosis and osteoporosis-related fractures are among the leading public health problems worldwide and are common in the geriatric population. Osteoporosis is a progressive metabolic bone disease characterized by low bone mass, microarchitectural deterioration, and decreased bone strength.1) It causes significant morbidity and mortality, particularly in geriatric patients. In addition to imaging methods for the diagnosis of osteoporosis, many biochemical markers have been investigated; however, no consensus has been reached regarding specific markers.
Matrix metalloproteinases (MMPs) were first identified in 1962 by Gross and Lapiere.2) MMPs are enzymes responsible for the degradation of extracellular matrix proteins during growth, normal tissue formation, organogenesis, and angiogenesis and play an important role in the regulation of intercellular communication and immunity.3) MMP2 and MMP9 are two leading MMPs that influence bone development and homeostasis.4) MMP activity is regulated by specific tissue inhibitors of metalloproteinases (TIMPs) that bind to MMPs and inhibit their functions.5) The balance between MMPs and TIMPs is important for maintaining bone quality. Although it is accepted that the shift of the balance between MMPs and TIMPs in the direction of MMP activity causes the destruction of the matrix and pathophysiological events such as atherosclerosis, cardiovascular diseases, inflammatory diseases, cancers, and osteoporosis occur, there remains confusion about this issue.6)
Osteoporosis, which is considered a geriatric syndrome, has been placed in a separate class by others. Geriatric syndromes such as malnutrition, cognitive impairment, dependence on basic and instrumental activities of daily living (BADL and IADL), and depression may have a common pathogenesis, as they are considered clinical conditions with common risk factors. If a relationship between osteoporosis and other geriatric syndromes can be found, common pathophysiological pathways can be revealed, and existing uncertainties can be clarified.7)
We searched for the terms, “osteoporosis” and “geriatric syndromes” together in the PubMed search engine to identify studies that investigated the relationship of osteoporosis with only one of the geriatric syndromes or that accepted osteoporosis as a geriatric syndrome. However, no study has been conducted so far that comprehensively and collectively evaluated the relationship between osteoporosis and several geriatric syndromes. In addition, we found few studies on the use of the MMP/TIMP ratio in osteoporosis, and the results were inconsistent. We comprehensively evaluated the relationship between osteoporosis and geriatric syndromes and investigated the use of MMP2/TIMP2 and MMP9/TIMP1 ratios as biomarkers of bone resorption in geriatric osteoporosis.
Our study sample consisted of 41 osteoporotic and 46 non-osteoporotic (23 with osteopenia) individuals aged 65 years and older who were admitted to the geriatric outpatient clinic of a university hospital. The sample was chosen using a non-probability consecutive sampling method. Although we divided the patients into groups with and without osteoporosis, we also divided the same patient population into three groups—osteoporosis, osteopenia, and control—and performed a subgroup analysis. Patients with osteopenia were included in the non-osteoporosis group as they did not meet the diagnostic criteria for osteoporosis. Informed consent was obtained from all participants. The study protocol was approved by the Local Ethical Review Committee of İstanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine (No. 2019-22507). Also, this study complied the ethical guidelines for authorship and publishing in the Annals of Geriatric Medicine and Research.8)
The study included patients aged 65 and above who underwent comprehensive geriatric assessment, underwent dual-energy X-ray absorptiometry (DEXA) in the last 1 month, and did not meet the exclusion criteria.
Patients <65 years of age with advanced organ failure and pathologies that may cause secondary osteoporosis, patients for whom the DEXA device could not be used, and patients who did not want to participate in the study despite meeting the appropriate criteria were excluded.
All patients underwent a comprehensive geriatric assessment. The Katz BADL and Lawton–Brody IADL scales were used to assess the independence of the study participants. The Katz BADL scale scores range between 0 and 27, with higher scores indicating increasing patient dependence.9) On the Lawton–Brody IADL scale, patients are scored between 0 and 17, with decreasing scores indicating increased patient dependence.10) The Mini-Nutritional Assessment (MNA) long-form was used to evaluate malnutrition, in which a score <23.5 was considered at risk for malnutrition.11) The Mini-Mental State Examination (MMSE) was used to screen cognitive function, with scores below 24 points considered indicative of cognitive dysfunction.12) The Geriatric Depression Scale (GDS) short form was used to identify depression possibility, with scores ≥5 points indicating possible depression.13)
DEXA was used to diagnose osteoporosis. Expert radiotechnologists measured bone mineral density (BMD) using the Hologic QDR 4500 Elite (Hologic Inc., Bedford, MA, USA) instrument. The precision expressed as the coefficient of variation (CV, %) was 1.6 at the total hip and 1.9 at the lumbar spine. These values were calculated after BMD measurements were performed twice for each of the 30 patients. The total femur or femoral neck T-scores were used to evaluate femoral measurements, and total (L1–L4) or at least two vertebral scores were used for vertebral measurements. Patients with a T-score below -2.5 standard deviation (SD) on DEXA were included in the osteoporosis group, those between -1 and -2.5 in the osteopenia group, and those above -1 SD in the control group.14) BMD values of the total femur, femoral neck, and lumbar spine (L1–L4) were noted.
We recorded calcium, phosphorus, albumin, parathyroid hormone, and 25-hydroxyvitamin D (25(OH)D3) levels. To measure MMP2, TIMP2, MMP9, and TIMP1 levels, blood was drawn from all participants between 08:00 and 09:00 after at least 8 hours of fasting. Blood was first transferred into an 8 mL tube without any additives or gel and centrifuged at 2,000–3,000 RPM for 15 minutes. The samples were stored at -80°C until analysis. MMP2, TIMP2, MMP9, and TIMP1 levels were determined by solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) using commercial kits (Bioassay Technology Laboratory, Beijing, China). For these parameters, the intra- and inter-CVs were <8% and <10%, respectively. The detection limits were as follows: MMP2, 5.6–3000 ng/mL; TIMP2, 2.4–1800 pg/mL; MMP9, 15.1–9000 ng/mL; and TIMP1, 0.23–200 ng/mL. The kit inserts were followed to examine the analytes in our study. Geriatric and biochemical physicians performed the preanalytical process.
A power analysis using G*Power version 3.1.9.6 (http://www.gpower.hhu.de) showed an effect size of 0.63 (<0.7 is an acceptable limit). The number of samples required to reach 80% power with “alpha error of 0.05” values was 41/45. We enrolled 41/46 (total 87) patients. Chi-square or Fisher exact tests were used for categorical variables. Student t-test or Mann–Whitney U test was used for continuous variables. Continuous variables are presented as mean±SD (if normally distributed) or median values with interquartile range (IQR) (if not normally distributed). This study analyzed the associations between osteoporosis and age, sex, body mass index, MMP9/TIMP1, and MMP2/TIMP2 using the univariate logistic regression (LR), and associations are reported as odds ratio (OR) with 95% confidence interval. Spearman correlation analysis was performed for the MMP/TIMP ratio and BMD values. Statistical significance was set at p<0.05. IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA) was used to analyze the clinical data.
Our study included 41 patients with osteoporosis (26 women) and 46 without osteoporosis (28 women). The mean age of the osteoporosis and control groups did not differ significantly (77.68±6.39 vs. 76.10±6.19 years; p=0.228). The BADLs scores were significantly higher and the IADLs scores were significantly lower in the osteoporosis group (p=0.001 and p=0.007, respectively). We observed no significant differences between the two groups in terms of MNA, GDS, and MMSE scores (p=0.598, p=0.898, and 0.287, respectively). On laboratory examination, calcium, phosphorus, parathyroid hormone, albumin, or 25(OH)D3 levels did not differ significantly between the two groups (p=0.251, p=0.528, p=0.682, p=0.082, and p=0.356, respectively) (Table 1).
The median MMP2/TIMP2 ratio of the group without osteoporosis was higher, but the difference was not significant (p=0.569). Likewise, while the median MMP9/TIMP1 ratio of the osteoporosis group was higher than that of the non-osteoporosis group; the difference was not significant (p=0.125). Similarly, we observed no significant differences between the two groups in MMP2, TIMP2, MMP9, or TIMP1 levels (p=0.538, p=0.912, p=0.718, and p=0.377, respectively). A detailed analysis is presented in Table 2.
Analysis of the association of osteoporosis with age, sex, body mass index, and MMP9/TIMP1 and MMP2/TIMP2 ratios using univariate LR showed no statistically significant association. The results of the LR analysis, including ORs, are presented in Table 3.
We analyzed the correlation of MMP/TIMP ratios with femur, femoral neck, and lumbar spine BMD using Spearman correlation analysis, the results of which are shown in Table 4. We observed no statistically significant correlation between the MMP9/TIMP1 and MMP2/TIMP2 ratios and BMD values of the femur, femoral neck, and lumbar spine.
As men and women have different levels of sex hormones and associations with bone metabolism, we performed separate analyses for both sexes. We observed no significant differences in MMP9, TIMP1, MMP2, TIMP2, MMP9/TIMP1, and MMP2/TIMP2 levels between the osteoporosis, osteopenia, and control groups in either sex, with p-values of 0.588, 0.733, 0.733, 0.436, 0.965, and 0.512 in women and 0.824, 0.267, 0.739, 0.076, 0.375, and 0.059 in men, respectively.
We also divided the same patient population into three groups—osteoporosis (n=41), osteopenia (n=23), and control (n=23)—and performed a subgroup analysis. We observed no significant differences among the three groups in terms of age (p=0.403). The median MMP2/TIMP2 ratios were 9.72 (IQR, 8.05–11.93) in the osteoporosis group, 10.74 (IQR,7.71–13.14) in the osteopenia group, and 10.34 (IQR,6.45–13.33) in the control group, with no statistically significant difference between these three groups (p=0.743) (Fig. 1). The median of the MMP9/TIMP1 ratio was 4.51 (IQR, 2.85–8.25) in the osteoporosis group, 3.29 (IQR, 2.29–6.91) in the osteopenia group, and 3.90 (IQR, 2.13–7.71) in the control group, with no significant difference (p=0.277) (Fig. 2).
Based on the knowledge that MMPs cause bone resorption and that TIMPs inhibit it, we hypothesized that MMP/TIMP ratios would increase in geriatric patients with osteoporosis. In addition to investigating the utility of MMP2/TIMP2 and MMP9/TIMP1 ratios as biomarkers of bone resorption in geriatric osteoporosis, we revealed the relationship between osteoporosis and geriatric syndromes and its effect on the dependence status of patients in both BADL and IADL.
Independence in older adults is important for their quality of life. In musculoskeletal diseases, the functionality of older adult patients decreases, and the patients become dependent on ADL. The results of a study conducted on 3,097 community-dwelling participants with musculoskeletal diseases, including osteoporosis, support this issue.15) The Irish Longitudinal Study on Aging (TILDA) investigated factors associated with impairment of BADL and IADL in community-dwelling older adults, in which both BADL and IADL were significantly affected in individuals with chronic conditions such as osteoporosis.16) Similarly, in our study, patients with osteoporosis were more dependent in both BADLs and IADLs (p=0.001 and p=0.007, respectively).
Depression is a geriatric syndrome with high prevalence. Studies have demonstrated the complex relationship between osteoporosis and depression. Although osteoporosis can cause depression, depression can also cause osteoporosis.17) Chronic pain, deterioration of physical ability, loss of self-esteem, and decreased quality of life caused by osteoporosis increase the prevalence of depression.18) In our study, although the GDS scores were higher in the osteoporosis group, the difference was not statistically significant (p=0.287). This may be because the GDS evaluates the possibility of depression and does not make a clear diagnosis; rather, depression is diagnosed based on clinical evaluation.
Since this study did not include patients diagnosed with secondary osteoporosis, and malnutrition was a secondary cause of osteoporosis, as expected, we observed no significant difference in MNA scores between the osteoporosis and control groups (p=0.598).
The activation of MMPs and their release from healthy tissues are limited; however, since various hormones, growth factors, and proinflammatory cytokines increase MMP activation, a significant increase in MMP levels is observed in pathologies that cause uncontrolled tissue destruction.3) MMPs and TIMPs have been investigated in many diseases, ranging from atherosclerosis to nephrolithiasis, cancer to periodontal disease, and diabetes to rheumatological diseases, most of which are associated with inflammation. A study investigating the roles of MMP1, MMP2, MMP9, and their tissue inhibitors in head and neck cancer reported that an imbalance between MMPs and their inhibitors played an important role in the progression of head and neck cancer and patient prognosis.19) Serum MMP2 and TIMP2 levels are elevated in antineutrophilic cytoplasmic antibody (ANCA)-associated vasculitis and chronic kidney disease.20,21) A review mentioned that MMP9 is secreted from neutrophils, macrophages, lymphocytes, and fibroblasts and contributes to cardiac remodeling by participating in both the early and late phases of post-myocardial infarction. The same review emphasized that MMP9 also affects other inflammatory diseases.22) We found no significant differences in MMP2, TIMP2, MMP9, and TIMP1 levels between the osteoporosis and control groups (p=0.538, p=0.912, p=0.718, and p=0.377, respectively). The lack of significance of the biomarkers examined in geriatric osteoporosis may be because it is not yet clear whether osteoporosis is an inflammatory condition. In addition, the exclusion of inflammatory conditions that may have caused secondary osteoporosis may have had an effect.
MMP2 and MMP9 play important roles in bone turnover.4) In our study, serum MMP2 and MMP9 levels were higher in the osteoporosis group than in the control group; however, the difference was not statistically significant (p=0.538 and p=0.718, respectively). This may be owing to the small sample size. Therefore, studies with larger numbers of patients are required.
While MMP consumption is generally believed to cause a loss of bone mass and because TIMP overexpression causes an increase in bone mass, some studies have shown that MMPs and TIMPs have independent effects on bone.5) The serum levels of MMPs and the balance between MMPs and TIMPs in osteoporosis have been the subjects of interest for researchers. Zhao et al.23) reported significantly higher MMP9 mRNA expression in osteoporotic bone tissues than in the control group. Similarly, Zhang et al.24) investigated the relationship between circulating MMP9 levels and osteoporosis in chronic obstructive pulmonary disease and found higher serum levels of MMP9 and MMP9/TIMP1 ratios in the presence of osteoporosis. In a rat study, MMP2 and MMP9 expression levels were negatively correlated with BMD.25) In our study, we found no significant differences in serum MMP2, TIMP2, MMP9, and TIMP1 levels, and MMP2/TIMP2 and MMP9/TIMP1 ratios between the osteoporosis, osteopenia, and control groups. The lack of difference in MMP/TIMP ratios between the osteoporosis and control groups may be because TIMPs are not the only regulators of MMPs; other proteins, such as transforming growth factor-β, bone morphogenetic protein, and Wnt/β-catenin, also interact with MMPs.26)
The results of our study showed no significant differences in blood MMP and TIMP levels and ratios between male and female patients with osteoporosis, osteopenia, and control patients. Collazes et al. investigated the presence of sex differences in various MMP and TIMP levels in patients with sepsis, stroke, and trauma and found that only MMP3 plasma levels were significantly higher in men than in women in each diagnostic group. Similar to our osteoporosis study, the authors found no differences in MMP2, MMP9, TIMP1, or TIMP2 levels between sexes.27) Experimental studies in human and rat cells have suggested that sex hormones play a small but definite role in the secretion of MMPs and TIMPs. However, the complexity of mediators and the strong effects of stimulatory and inhibitory pathways caused by concomitant diseases or infections may minimize the sex effects.
Our study had some limitations. The first is the absence of young patients with osteoporosis and young controls. Second, including bone resorption markers such as C-telopeptide would have been ideal, while examining the relationship between serum levels and the ratios of MMPs and TIMPs and bone resorption in geriatric osteoporosis. However, owing to the conditions in our country, we could not compare with other markers because we were not provided with more funds.
The strength of our study is that it is the first to comprehensively examine the relationship between osteoporosis and several geriatric syndromes, as well as the relationship between osteoporosis and serum MMP, TIMP values, and MMP/TIMP ratios in geriatric patients.
In conclusion, as found in our study, geriatric individuals with osteoporosis became more dependent on both BADLs and IADLs. Therefore, early intervention for the disease is important. In addition, our results showed that the MMP2/TIMP2 and MMP9/TIMP1 ratios did not provide additional benefits in demonstrating bone resorption in geriatric osteoporosis. However, this topic should be examined in future studies with larger numbers of patients. New biomarker studies are required to elucidate the diagnosis of osteoporosis in geriatric patients.
ACKNOWLEDGMENTS
Fig. 1.
MMP2/TIMP2 ratio of the control, osteopenia, and osteoporosis groups. MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
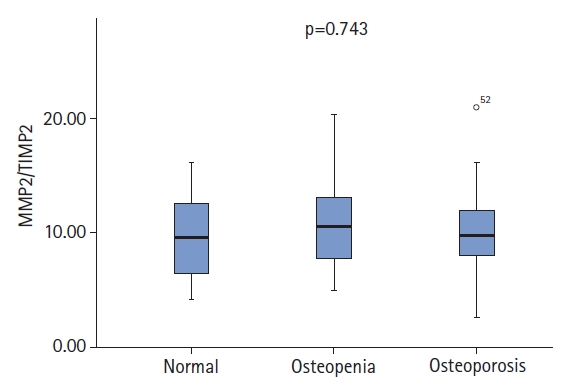
Fig. 2.
MMP9/TIMP1 ratio of the control, osteopenia, and osteoporosis groups. MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
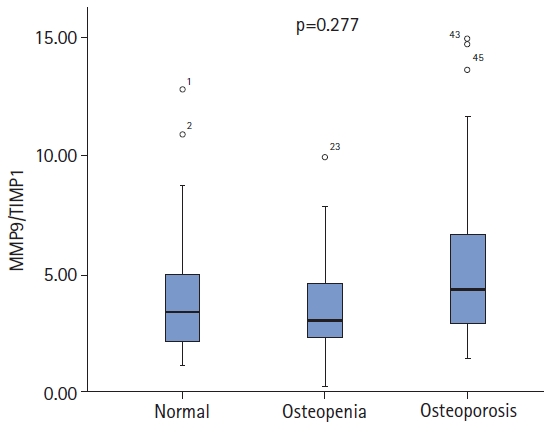
Table 1.
Demographic and clinical characteristics and laboratory findings of patients with and without osteoporosis
| Osteoporosis (n=41) | Non-osteoporosis (n=46) | p-value | |
|---|---|---|---|
| Sex | 0.807 | ||
| Female | 26 | 28 | |
| Male | 15 | 18 | |
| Age (y) | 77.68±6.39 | 76.10±6.19 | 0.228 |
| BMI (kg/m2) | 27.94±6.60 | 28.89±4.93 | 0.122 |
| BADLs | 4.91±1.22 | 3.87±1.55 | 0.001* |
| IADLs | 4.21±2.09 | 5.40±1.87 | 0.007* |
| Mini-Mental State Examination | 24.7±3.2 | 24.6±2.9 | 0.898 |
| Mini-Nutritional Assessment | 23.8±3.2 | 24.2±2.80 | 0.598 |
| Geriatric Depression Scale | 3.42±2.46 | 2.88±2.29 | 0.287 |
| Calcium (mg/dL) | 9.03±0.55 | 8.87±0.69 | 0.251 |
| Phosphorus (mg/dL) | 3.41±0.48 | 3.47±0.47 | 0.528 |
| Albumin (g/dL) | 3.71±0.46 | 3.89±0.48 | 0.082 |
| Parathormone (pg/mL) | 49.4±23.3 | 51.5±22.7 | 0.682 |
| 25(OH)D3 (ng/dL) | 22.0±12.5 | 19.8±9.9 | 0.356 |
| Femur | |||
| T-score | -1.99±0.71 | -0.78±0.72 | <0.001* |
| BMD (g/cm²) | 0.652±0.098 | 0.792±0.138 | <0.001* |
| Femoral neck | |||
| T-score | -2.40±0.47 | -1.13±0.54 | <0.001* |
| BMD (g/cm²) | 0.566±0.064 | 0.739±0.084 | <0.001* |
| Lumbar spine (L1-L4) | |||
| T-score | -2.25±0.62 | -0.92±0.84 | <0.001* |
| BMD (g/cm²) | 0.792±0.077 | 0.906±0.083 | <0.001* |
Table 2.
Serum MMP, TIMP levels, and MMP/TIMP ratios of patients with and without osteoporosis
Table 3.
Univariate LR analysis of the factors predicting osteoporosis in geriatric patients
|
Univariate LR |
||
|---|---|---|
| OR (95% CI) | p-value | |
| MMP2/TIMP2 | 1.033 (0.913–1.168) | 0.608 |
| MMP9/TIMP1 | 1.013 (0.931–1.102) | 0.765 |
| Age | 1.092 (0.994–1.200) | 0.067 |
| BMI | 1.014 (0.126–8.156) | 0.990 |
| Sex | 0.844 (0.277–2.574) | 0.765 |
REFERENCES
1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785–95.


2. Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A 1962;48:1014–22.



4. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463–516.



5. Paiva KBS, Granjeiro JM. Matrix metalloproteinases in bone resorption, remodeling, and repair. Prog Mol Biol Transl Sci 2017;148:203–303.


6. Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol 2004;49:187–98.


7. Moon S, Roh YK, Yoon JL, Jang KU, Jung HJ, Yoo HJ, et al. Clinical features of geriatric syndromes in older Koreans with diabetes mellitus. Ann Geriatr Med Res 2019;23:176–82.




8. Noh JH, Jung HW, Ga H, Lim JY. Ethical guidelines for publishing in the Annals of Geriatric Medicine and Research. Ann Geriatr Med Res 2022;26:1–3.




9. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185:914–9.


10. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86.


11. Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999;15:116–22.


12. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.

14. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359–81.




15. Stamm TA, Pieber K, Crevenna R, Dorner TE. Impairment in the activities of daily living in older adults with and without osteoporosis, osteoarthritis and chronic back pain: a secondary analysis of population-based health survey data. BMC Musculoskelet Disord 2016;17:139.




16. Connolly D, Garvey J, McKee G. Factors associated with ADL/IADL disability in community dwelling older adults in the Irish longitudinal study on ageing (TILDA). Disabil Rehabil 2017;39:809–16.


17. Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T. The association of bone mineral density and depression in an older population. J Am Geriatr Soc 2001;49:732–6.


18. Aloumanis K, Mavroudis K. The "depressive" face of osteoporosis and the "osteoporotic" face of depression. Hormones (Athens) 2013;12:350–62.



19. Pietruszewska W, Bojanowska-Pozniak K, Kobos J. Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: an immunohistochemical study. Otolaryngol Pol 2016;70:32–43.


20. Zakiyanov O, Chocova Z, Hruskova Z, Hladinova Z, Kalousova M, Malickova K, et al. Matrix metalloproteinases and their tissue inhibitors: an evaluation of novel biomarkers in ANCA-associated vasculitis. Folia Biol (Praha) 2019;65:227–36.


21. Kobusiak-Prokopowicz M, Krzysztofik J, Kaaz K, Jolda-Mydlowska B, Mysiak A. MMP-2 and TIMP-2 in patients with heart failure and chronic kidney disease. Open Med (Wars) 2018;13:237–46.



22. Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 2013;139:32–40.



23. Zhao H, Cai G, Du J, Xia Z, Wang L, Zhu T. Expression of matrix metalloproteinase-9 mRNA in osteoporotic bone tissues. J Tongji Med Univ 1997;17:28–31.



24. Zhang PF, Pan L, Luo ZY, Zhao HJ, Cai SX. Interrelationship of circulating matrix metalloproteinase-9, TNF-α, and OPG/RANK/RANKL systems in COPD patients with osteoporosis. COPD 2013;10:650–6.


25. Zheng X, Zhang Y, Guo S, Zhang W, Wang J, Lin Y. Dynamic expression of matrix metalloproteinases 2, 9 and 13 in ovariectomy-induced osteoporosis rats. Exp Ther Med 2018;16:1807–13.



- TOOLS







