Korean Working Group on Sarcopenia Guideline: Expert Consensus on Sarcopenia Screening and Diagnosis by the Korean Society of Sarcopenia, the Korean Society for Bone and Mineral Research, and the Korean Geriatrics Society
Article information
Abstract
Despite the introduction of a diagnostic code and acceptance of a diagnostic process for sarcopenia as a new health technology in Korea, many practitioners remain unfamiliar with the evaluation of sarcopenia. Thus, the Korean Working Group on Sarcopenia (KWGS) developed clinical practice guidelines for the diagnosis of sarcopenia in older Korean adults. A two-phase Delphi interview comprising 19 questions was conducted with 40 expert panelists, 22 of whom participated in the first round between June and August 2022. The second round of the Delphi interview included the remaining 11 questions that were not agreed upon in the first round. The screening process for sarcopenia includes various questionnaires and examinations used in different research and clinical settings. The diagnostic process for sarcopenia was simplified by combining the steps of case finding and assessment. The Short Physical Performance Battery test was given particular emphasis owing to its multifaceted nature. Regardless of muscle mass, having low muscle strength with low physical performance is considered clinically relevant and newly defined as “functional sarcopenia.” Comprehensive geriatric assessment is important for diagnosing sarcopenia. The KWGS’s clinical guideline aims to facilitate the early detection of sarcopenia by allowing various screening tools to be used in a unified process and reducing confusion about which tools to use for diagnosis. This recommendation expands the conceptual definition of sarcopenia as a complex pathophysiological state in line with the concept of frailty and aims to stimulate further research on the diagnosis and management of sarcopenia in clinical settings.
INTRODUCTION
Sarcopenia is an age-related condition characterized by decreased muscle mass and impaired muscle strength or physical performance.1) The prevalence of sarcopenia increases with age, ranging from 5.5%–25.7% in community-dwelling older adults in Asian countries according to the Asian Working Group for Sarcopenia (AWGS) 2014 criteria.2,3) Korean studies have reported a 4%–45% prevalence of sarcopenia in older adults using various definitions.4)
The clinical relevance of sarcopenia is increasing because it disproportionately affects older and vulnerable populations. The clinical outcomes include the progression of frailty, incidence of falls and fractures, further functional impairment, resultant institutionalization, and death.5-7) Many Asian countries are experiencing rapid population aging. Considering its overarching effects on health outcomes and quality of life in older adults, sarcopenia is increasingly recognized as a clinical disease. For instance, the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10 CM) included sarcopenia as a clinical condition in 2016,8) and the revised Korean Standard Classification of Diseases-8 (KCD-8) also included sarcopenia as a clinical condition in 2021, with diagnostic code M62.5.
Unlike many clinical conditions, including malignancies and genetic disorders, with major molecular drivers commonly targeted as mechanisms of action in new drug development, sarcopenia has a more complicated pathophysiology as a human aging phenotype or geriatric syndrome.7,9) The direct contributors adversely affect the homeostatic maintenance of muscle mass and function, including decreased mechanical inputs, insufficient nutritional stimuli, and biological alterations, resulting in increased muscle catabolism and decreased net muscle protein synthesis due to mechanical and nutritional inputs (anabolic resistance).10-13) Myriad geriatric clinical and functional conditions directly or indirectly affect these contributors. Sarcopenia is a multifaceted condition with extensive effects on various geriatric health domains. It retro-reflectively modulates functional states in these domains, such that sarcopenia impairs the ability of older adults to function optimally in diverse aspects of life, including activities of daily living and cognitive function (Fig. 1). Thus, given the complex nature of sarcopenia, its definition cannot be captured by a single biomarker or clinical features. Such a simplified approach is unlikely to encompass the full complexity of the condition and the underlying pathophysiological mechanisms. Therefore, a more comprehensive strategy is required to understand and manage sarcopenia in the older adult population.
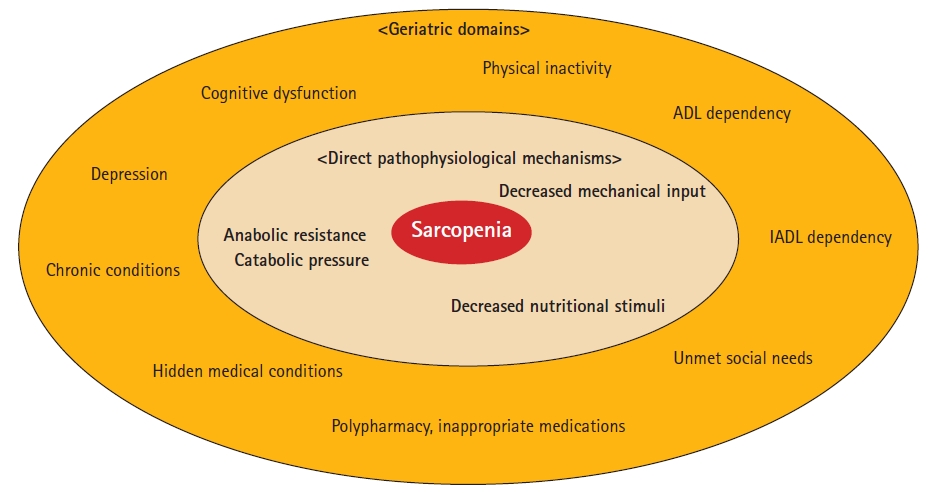
Sarcopenia is a complex system consisting of numerous pathophysiologies in both biological and clinical aspects. ADL, activities of daily living; IADL, instrumental activities of daily living.
Historically, the operational definitions of sarcopenia have evolved to capture its characteristics as a geriatric syndrome, reflecting longitudinal studies of muscle-related parameters and age-related outcomes. Using the same approach to defining osteoporosis, sarcopenia was initially defined using muscle mass parameters based on the population distribution.14,15) As accumulating studies show the clinical relevance of functional parameters such as grip strength or low-extremity physical performance over muscle mass, the classification of sarcopenia has gradually better captured the components of physical frailty.16) For instance, in 2010, the original European Working Group on Sarcopenia in Older People (EWGSOP) defined sarcopenia using muscle mass and function17) and subsequently published consensus guidelines, including the Foundation for the National Institutes of Health (FNIH),18) AWGS in 2014,2) AWGS in 2019,19) and EWGSOP2, which generally followed a similar approach to operationalization. Notably, recent statements from the Sarcopenia Definitions and Outcomes Consortium (SDOC)20) and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO) emphasized the importance of muscle function over muscle mass, both in determining sarcopenia and measuring clinical improvements, especially in intervention studies.21)
Korea is one of the fastest-aging countries worldwide. As the baby-boomer population born from 1955 to 1963 is entering elderhood, Korea is expected to experience further growth in terms of care needs for older people with functional impairment in the coming years, making the prevention, early recognition, and intervention of sarcopenia in the Korean population an imminent issue, if not already overdue. However, despite the importance of this condition, consensus guidelines on sarcopenia in the Korean population have not yet been established. As different populations, healthcare systems, and sociocultural factors may affect the diagnosis of sarcopenia, we aimed to provide an expert consensus on the diagnosis of sarcopenia in Korean community-dwelling older adults.
CONSENSUS PROCESS
The Korean Working Group on Sarcopenia (KWGS) guidelines were established in 2019 based on the liaison efforts of the Korean Society of Sarcopenia, the Korean Society for Bone and Mineral Research, and the Korean Geriatrics Society to provide a nation-specific consensus on sarcopenia. Following email discussions, the KWGS held six online expert brainstorming meetings to develop key questions for the Delphi interview processes to establish a Korean consensus on sarcopenia diagnosis, starting in March 2022.
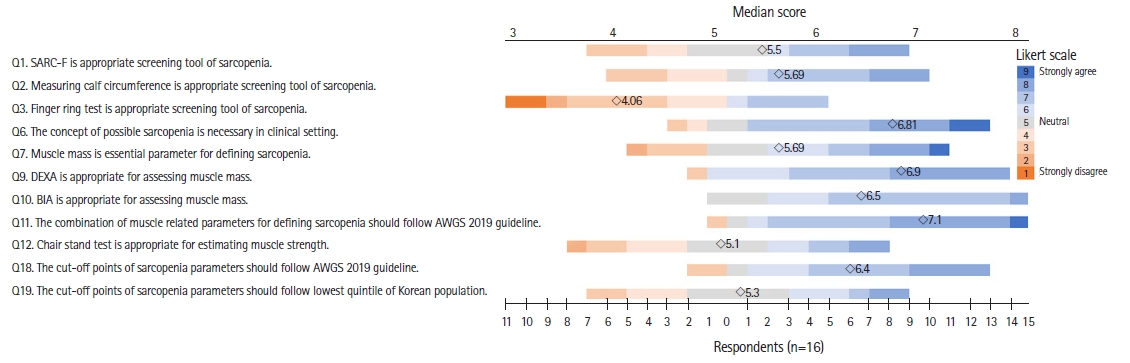
The Delphi interview questionnaire was designed to bridge the gap between clinical environments and research evidence from the Korean population and existing international guidelines currently in use in the country, and included 19 questions for the first round (Fig. 2). In the interviews, we used a scale from 1 (strongly disagree) to 9 (strongly agree) to measure consensus for 15 single-choice questions. The remaining four questions were multiple-choice with no restrictions on the number of answers (Fig. 3).
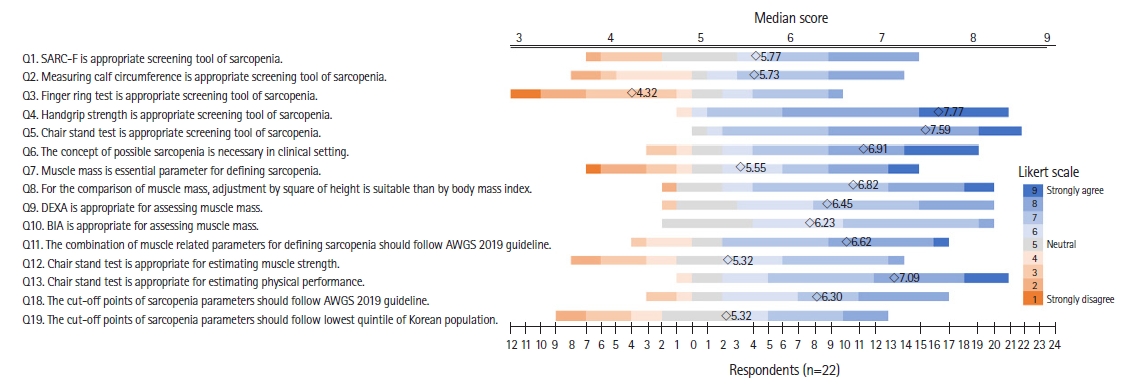
Specific questions and the level of agreement in the first Delphi round. SARC-F, strength, assistance with walking, rising from a chair, climbing stairs, and falls; DEXA, dual-energy X-ray absorptiometry; BIA, bioimpedance analysis; AWGS, Asian Working Group for Sarcopenia.
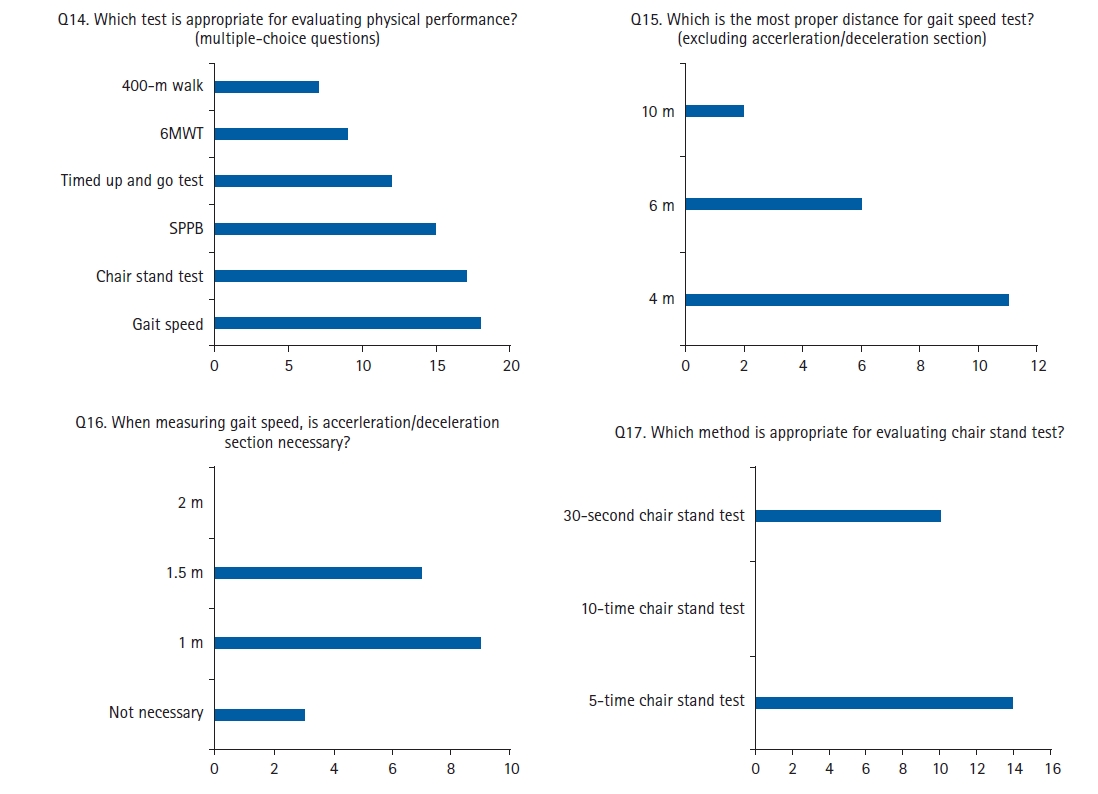
Multiple-choice questions of the first Delphi round. 6MWT, 6-minute walk test; SPPB, short physical performance battery.
For the Delphi interview, the questions were sent via email to 40 panelists who were experts in geriatric medicine, endocrinology, physical medicine, rehabilitation, oncology, orthopedic surgery, family medicine, exercise physiology, nutrition, healthcare policy, and industry. A total of 22 panelists—5 geriatricians, 4 endocrinologists, 3 rehabilitation specialists, 2 family medicine practitioners, 2 physical medicine experts, 1 exercise physiology specialist, 1 orthopedist, 1 oncologist, 1 nutrition specialist, 1 healthcare policy expert, and 1 healthcare industry expert—responded to the first round of interviews. In the first round, four of the 15 single-choice items reached agreement among the respondents (Fig. 2). We proceeded to the second round with the 11 remaining items for which we did not reach an agreement in the first round (Fig. 4). Fifteen panelists participated in the second round. To quantify the degree of agreement, we used the content validity ratio (CVR), with CVRs of 0.39 for the first round and 0.49 for the second round, considering the number of respondents.22) Of these 11 questions, six demonstrated a high level of agreement among the experts, ultimately resulting in a consensus. For the remaining five questions, the KWGS members determined that additional rounds would not substantially affect the outcome or level of agreement among the experts. From two rounds of Delphi interviews, KWGS consensus recommendations were drafted and further reviewed by board members. A draft of the recommendations was presented at the 13th Congress of the Korean Society of Sarcopenia in December 2022.
SUMMARY OF THE ASSESSMENT FLOW FOR SARCOPENIA
The general operational classification flow for sarcopenia suggested by the KWGS is shown in Fig. 5. For case finding, the range of tools consists of questionnaires such as the SARC-F, while the examinations include calf circumference, finger ring, hand grip, chair stand, gait speed, and timed up-and-go (TUG) tests. Experts agree that various screening methods can be used for specific research or practice settings. For instance, questionnaires may be adopted with minimal additional workload, albeit with some drawbacks in sensitivity or specificity in the Korean population,23) at least in mass-scale community-based studies or public health examinations. We maintained the concept of possible sarcopenia, similar to the EWGSOP2 and AWGS 2019.5,19) In contrast to the AWGS 2019, we combined case finding and assessment in one step to simplify the classification flow.
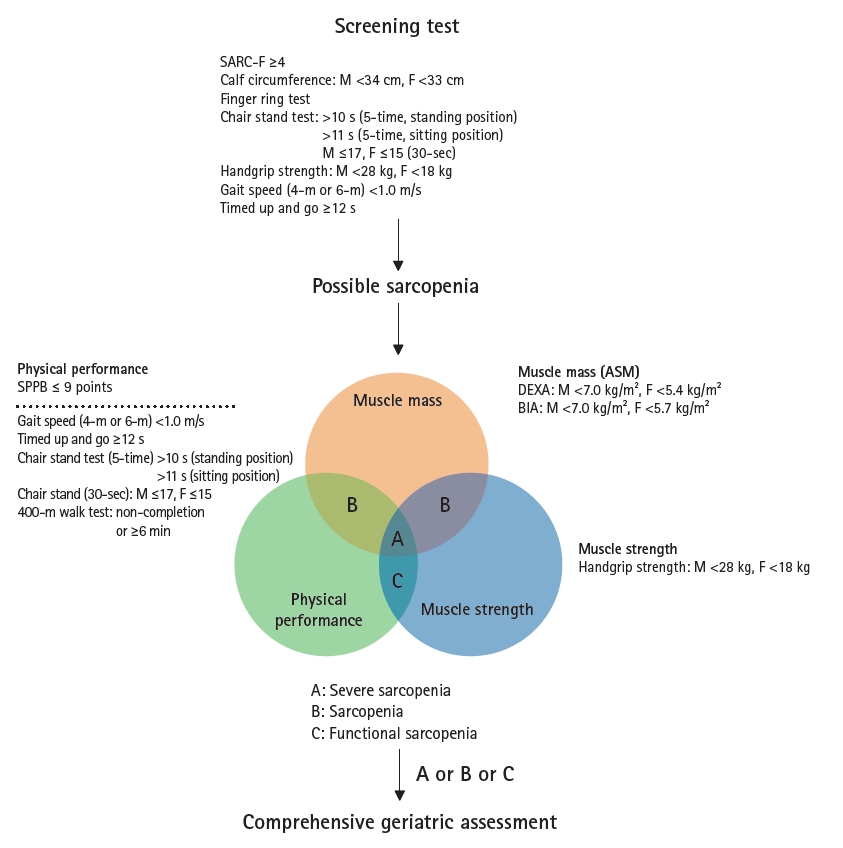
Algorithm for sarcopenia evaluation. SARC-F, strength, assistance with walking, rising from a chair, climbing stairs, and falls; SPPB, short physical performance battery; ASM, appendicular skeletal muscle mass; BIA, bioimpedance analysis; DEXA, dual-energy X-ray absorptiometry.
To confirm the presence of sarcopenia, we recommend an evaluation that includes the following three parameters: appendicular skeletal muscle mass (ASM), muscle strength, and physical performance. We defined “sarcopenia” as decreased muscle mass with low muscle strength or poor physical performance. Severe sarcopenia was classified as a state of decreased muscle mass in the presence of both weak muscle strength and decreased physical performance. “Functional sarcopenia” was classified as a state of weak muscle strength and low physical performance without a loss of muscle mass, which deserves a similar intervention effort as sarcopenia as evidence supports the outcome relevance of this condition in older adults.
Since muscle loss is a phenotype arising from aggregated inputs of biological and functional pathophysiology, we recommend performing a comprehensive geriatric assessment (CGA) for patients with either “sarcopenia” or “functional sarcopenia” to identify interconnected geriatric conditions and establish person-specific strategies to prevent the progression of these conditions, as evidence supports the beneficial role of geriatric multicomponent interventions.24-26) Experts in the KWGS noted that the concepts of primary and secondary sarcopenia are less obvious in patients in real-world practice, and underlying clinical conditions affecting muscle homeostasis should be identified through CGA, if possible, or appropriate evaluations for evidence-based multicomponent interventions for sarcopenia.
SCREENING TESTS
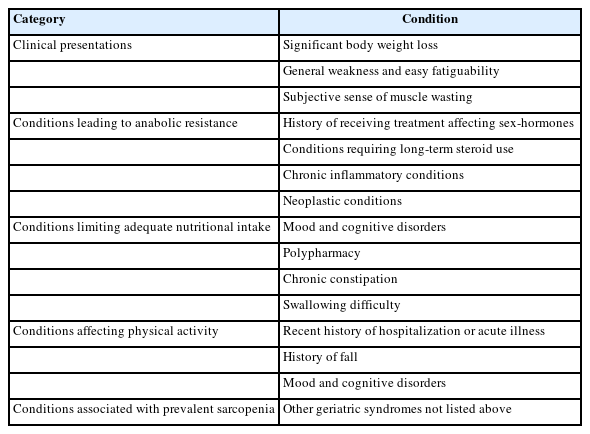
We recommend using any validated screening tools, as various screening instruments perform well in classifying sarcopenia in the Korean population.19,27-29) The eligible populations for sarcopenia evaluation included individuals aged ≥65 years, postmenopausal women <65 years, and younger adults with clinical presentations or a history of clinical conditions that affect muscle homeostasis (Table 1). Additionally, if there is clinical suspicion, screening can be skipped, and direct progress can be made toward diagnosis.
Consistent with existing guidelines, we recommend seven screening tools: SARC-F, calf circumference, finger-ring test, chair stand test, handgrip strength, gait speed, and TUG (Table 2). The SARC-F questionnaire consists of five components that evaluate strength, assistance in walking, rising from a chair, climbing stairs, and falling.30) Though a SARC-F score ≥4 has shown low to moderate sensitivity and high specificity in detecting sarcopenia with the possibility of recall bias in frail older adults with cognitive decline,31,32) it is still a good screening tool as it is simple and feasible without requiring advanced equipment; moreover, it is well-validated in many works of literature, including Korea.23,33,34) To apply the SARC-F to a massive population, such as in the community, lowering the cutoff score from 4 to 2 could improve the sensitivity.35,36) Calf circumference has shown moderate to high sensitivity and specificity in detecting sarcopenia, and its appropriate cutoffs are <34 cm and <33 cm in men and women, respectively.19) As calf circumference entails muscle mass rather than muscle function, it can be used with SARC-F and SARC-C.37-39) For an accurate and consistent assessment of calf circumference, measurement in a standing position with a non-elastic tape is recommended.36) Further adjustments for height, weight, or body mass index (BMI) might be considered to address the underdetection of sarcopenia in obese people.36,40) Likewise, the finger-ring test can be an alternative for calculating calf circumference by encircling the thickest part of the calf with both the thumbs and index fingers. Sarcopenia is suspected if the calf is thinner than the finger ring.41,42) Both handgrip strength and chair stand tests are also recommended in sarcopenia screening with the same cutoff for diagnosis—handgrip strength of men <28 kg and women <18 kg; chair stand test of >10 seconds (5-time, ending with standing position) and >11 seconds (5-time, ending with sitting position), or men ≤17 and women ≤15 (30 seconds).19,28,29) In addition, the gait speed test and TUG can be used as screening tools using the cutoff values of a gait speed of <1.0 m/s and TUG ≥12 seconds.19,43) In clinical settings with a high prevalence of sarcopenia, adopting handgrip strength, chair stand test, gait speed, or TUG test may simplify the diagnostic steps for sarcopenia.

Proposed tool for screening sarcopenia and assessing muscle mass, muscle strength, and physical performance
A recent surge in research interest in the opportunistic measurement of the psoas muscle cross-sectional area at the L3 lumbar vertebra or muscle volumetry methods using conventional or machine-learning algorithms allows the assessment of muscle mass in patients undergoing cross-sectional imaging studies for various medical or surgical purposes.44-47) Although most studies have focused on muscle mass-related parameters and clinical outcomes, patients with low muscle mass in these opportunistic imaging methods might be subjected to formal sarcopenia evaluations if muscle wasting is clinically suspected. However, the potential use of cross-sectional imaging as a screening tool should be further explored and evaluated in future studies.
MUSCLE MASS
Muscle quantity can be estimated by ASM measured using dual-energy X-ray absorptiometry (DEXA) and bioimpedance analysis (BIA), according to the recent AWGS 2019 guidelines.19)
DEXA uses lean mass, excluding bones, to indirectly estimate ASM and has been well validated as a standard method to assess muscle mass in many studies; however, connective tissues such as skin and blood vessels or the amount of body water could be counted as lean mass. Additionally, measurements can vary depending on the manufacturer’s brand, correction technique, and post-processing method.48-50) Using the difference in the electrical conduction rate between fat and water, BIA indirectly estimates the body fat and lean masses. BIA has several advantages; it is cost-effective, portable, easy to operate, and safe with no radiation exposure.51) However, concerns regarding its accuracy exist according to the examinee’s race, body water status, and BMI. This technology has gradually evolved from single-frequency to multi-frequency and from whole-body to segment-specific impedance, allowing the estimation of appendicular lean mass. Direct-segmental multi-frequency bioelectrical impedance analysis (DSM-BIA) has shown a good correlation with DEXA in estimating body composition and lean mass.52-54) In addition, a segmental index of extracellular water in proportion to the total body water (ECW/TBW) for excess fluid55-57) and a phase angle for estimating muscle quantity can be used adjunctly.58,59)
For the ASM adjustment method, the KWGS recommends a squared value of height (m2) according to the AWGS 2019. Additionally, the BMI-adjusted ASM can be used to capture sarcopenia in obese individuals.18) The recommended cutoff points for height-adjusted ASM follow those in the AWGS 2019: <7.0 kg/m2 (men) and <5.4 kg/m2 (women) in DEXA; <7.0 kg/m2 (men) and <5.7 kg/m2 (women) in BIA.19) The recommended cutoff points for BMI-adjusted ASM follow the FNIH: <0.789 (men) and <0.512 (women) for DEXA.18)
Although computed tomography and magnetic resonance imaging can also be used to assess muscle mass, their high costs and radiation exposure hinder their clinical use for mainly sarcopenia. Studies on opportunistically-acquired muscle mass parameters in cross-sectional imaging are insufficient in terms of compatibility between these parameters and lean mass from DEXA or BIA. The D3-creatine dilution method can be considered.60-62) Studies suggest that inconsistent associations between muscle mass and adverse health outcomes, including impaired mobility, disability, falls, and mortality, might be attributed to the indirect nature of DEXA and BIA, which measure lean mass rather than muscle mass per se (SDOC).20) Direct measurement methods may fill this gap by acquiring muscle mass. While regulatory protocols for adopting stable isotopes such as D3-creatine are still ongoing in the Ministry of Food and Drug Safety, as these compounds are considered experimental pharmaceuticals, we expect that the research use of direct muscle mass measurement will become popular in the future, especially for measuring objective intervention effects between before and after.
MUSCLE STRENGTH
Although both handgrip strength (upper extremities) and knee joint torque (lower extremities) can be measured, we recommend handgrip strength as a surrogate index of muscle strength owing to its accessibility for community-dwelling individuals, concordant with the major guidelines of sarcopenia. Unlike EWGSOP2, which uses the chair stand test as a proxy for the strength of the lower muscles,5) our expert group considered the chair stand test to be an indicator of muscle power (force×velocity) rather than strength (force) because it includes both velocity and strength (force). Additionally, the chair stand test represents complex physical performance, including balance, endurance, and coordination, and has shown a better association with physical performance parameters (e.g., gait speed) than with handgrip strength.63) Hence, we included the chair stand test in the physical performance section per the AWGS guidelines.19)
Both spring-type (Smedley) and hydraulic-type (Jamar) dynamometers can be used to assess handgrip strength; the examiner should follow the standard protocol for each type. As the hydraulic type tends to have higher test values than the spring type,64) the test values are not interchangeable. Thus far, separate cutoff values for each test are not provided in the AWGS 2019 owing to a lack of studies comparing the two methods of measuring handgrip strength.19) In a spring-type dynamometer, measurements should be performed in a standing position with the elbow extended.65) If the patient cannot maintain a standing position, a sitting position with the elbow extended is recommended.36) For the hydraulic type of dynamometer, grip strength is measured in a sitting position with the elbow flexed at 90°.65)
The measurement can be performed in both arms or the dominant arm at least twice, with the maximum value among all these examinations defined as the grip strength. There is no time limit for the assessment and encouraging the examinee to exert maximum effort is recommended. The cutoff value for low handgrip strength is <28 kg in men and <18 kg in women, as per the AWGS 2019 guidelines.19)
PHYSICAL PERFORMANCE
Among the different instruments available to evaluate physical performance, we recommend the Short Physical Performance Battery (SPPB) as a priority because it encompasses all three phenotypes of physical performance: gait speed, balance, and chair stand test. One or two additional tests can be used complementarily to determine the state of low physical performance. If the SPPB is not executable, gait speed or TUG test can be used as an alternative. The tests are prioritized to avoid the spuriously high prevalence of a low physical performance state by interpreting any of the many executed tests as positive.66) While deciding on an appropriate representative test based on the characteristics of individual institutions and clinical circumstances is essential, we recommend adopting up to two tests when classifying low physical performance.
The SPPB is appropriate for evaluating the functions of daily living as it comprises three basic components: usual gait speed, static balance, and five-time chair stand test. Additionally, the SPPB has been widely used as a primary prognostic factor to determine the point of sarcopenia intervention and its effectiveness in numerous clinical studies.21,67-69) For gait speed measurement, the participants are asked to walk 3 or 4 m at their usual pace. The balance test consists of side-by-side, semi-tandem, and tandem standing with the participant holding the position for at least 10 seconds. In the chair stand test, individuals are instructed to stand up from a chair five times without using their arms. Each component’s score ranges from 0 to 4, resulting in a total possible score of 12 points. The cutoff points for SPPB follow the AWGS 2019 guidelines (≤9 points).19)
Gait speed is a well-validated and reliable test to assess physical performance and has shown good correlation with sarcopenia-related outcomes, including mobility limitation, disability, falls, institutionalization, and death.70-73) Therefore, both 4 m and 6 m test lengths are recommended, with separate 1 m or 1.5 m acceleration and deceleration lengths. Our experts from the KWGS acknowledged the necessity of the acceleration and deceleration areas because discrepancies in this section can exist in participants with severe frailty with decreased attention or a mobility disorder such as Parkinson disease (Fig. 3). The optimal acceleration and deceleration lengths for gait speed measurement remain controversial, and instrumented measurements by sensors with high spatiotemporal resolution may reduce the space required for examination, as the mean velocity section can be selected by algorithms. The AWGS 2019 cutoff for gait speed is <1.0 m/s.19)
The TUG comprises the elements of getting up from a chair, turning the 3 m-halfway point at the usual pace, and sitting back on the same chair. Although the TUG is not advised as an index of physical performance in AWGS 2019, experts from the KWGS determined that the TUG can reflect multiple aspects of human health by containing segments of the SPPB, such as walking at the usual pace and rising from a chair, which are also essential for daily living. Since sarcopenia per se is a multicausal and complex system in line with the concept of frailty, the TUG can be a good surrogate marker of physical performance.5,74) Because a universal cutoff value for the TUG is lacking, we recommend a new cutoff value based on the study results of Korean community-dwelling older people.43) Given that the lowest quintile of TUG is ≥11.8 seconds for men and ≥12.5 seconds for women, we recommend a cutoff point of ≥12 seconds for both sexes.43)
The chair stand test times five consecutive rises from a chair as quickly as possible, with no assistance from either arm. In a community setting with relatively robust participants, a five-time chair stand test may have limited discrimination power. Thus, the 30-second chair stand test (counting the number of seconds spent rising from a chair) is recommended in this case.75) The two possible ways of measuring the time for a chair to stand are starting with a sitting position and finishing in a standing position, and starting with a sitting position and finishing in a sitting position. Both practical methods can be used if measured consistently.29,36) For the five-time chair stand test, the cutoff value is >10 seconds (five-time, ending in the standing position), >11 seconds (five-time, ending in the sitting position).29) For the 30-second chair stand test, the cutoff is ≤17 in men and ≤15 in women.28)
The 400-m walk test has the advantage of evaluating endurance and walking ability. Assessing how far individuals can walk out of their houses is critical as it is directly related to individual autonomy and quality of life. For this examination, we considered taking more than 6 minutes to be associated with decreased physical performance according to EWGSOP2.5)
Physical performance tests are traditionally performed manually using stopwatches and floor markings. However, recent advances in sensor technology have enabled the development of automatic devices that can capture human biomechanical parameters in these tests. Devices have been developed and validated for tests such as gait speed76) and other gait parameters,77) TUG test, chair stand test,78,79) and the SPPB.80)
CLINICAL IMPLICATIONS AND FUTURE DIRECTIONS
Although the sarcopenia diagnosis code has been introduced, and the diagnostic process based on muscle mass measurement using BIA and DEXA has been accepted as a new health technology in Korea, most practitioners remain unfamiliar with making diagnoses and selecting evaluation tools for sarcopenia in routine clinical practice. This unexpected discrepancy may be attributable to inconsistencies in our understanding of the biological or clinical constructs of sarcopenia. For instance, some researchers recognize sarcopenia as a phenotype of low muscle mass relative to fat mass, a metabolic condition rather than an age-related decrease in muscle health.81) Consequently, in our study, the selection of specific assessment tools for classifying sarcopenia was complicated by many key issues that did not reach convergence. This issue is further complicated by the tendency to approach sarcopenia as a single disease entity rather than as a problem of a complex system arising from human aging and frailty in the current Korean culture with disease-oriented, specialty-centered healthcare systems. In some instances, the intervention for sarcopenia is restricted to the domains of protein supplementation and one-size-fits-all-type exercises for logistical convenience,82) while the geriatric domains of multimorbidity, polypharmacy, cognitive decline, depression, and social care needs are often overlooked in sarcopenia assessment and intervention.
To address these problems, in the KWGS clinical practice guidelines, we highlight the following specific points. First, we include a diverse range of screening tools consisting of questionnaires and examinations for easier case finding in different research and clinical settings. Additionally, we combined the two existing steps suggested in EWGSOP2 and AWGS 2019—case-finding and assessment—into a single step to simplify the classification flow. Second, we prioritized the various tools for measuring physical performance, making the SPPB representative owing to its multifaceted composition, including gait speed, balance, and chair stand test. As such, we intended to minimize disagreements in test results from different measuring tools to avoid the overdetection of low physical performance. Third, apart from existing sarcopenia guidelines placing muscle mass as a pivotal parameter for defining sarcopenia, experts from the KWGS determined that having low muscle strength with low physical performance also has clinical relevance, even in the absence of decreased muscle mass. Thus, we define this state as “functional sarcopenia.” Finally, emphasizing sarcopenia as a geriatric mobility condition with a complex pathophysiology rather than as a single disease entity, we highlight the execution of CGA after making a final diagnosis of sarcopenia in our diagnostic flow.
The new KWGS clinical guidelines intend to facilitate the early detection of sarcopenia by permitting diverse screening tools with a unified process and reducing confusion in selecting diagnostic tools. With this recommendation, we hope to expand the conceptual definition of sarcopenia to a state of complex pathophysiology in line with the concept of frailty. Using this approach, we expect healthcare professionals to be able to design holistic, personalized intervention plans based on CGA, embracing multiple domains, not only nutrition and physical activity, but also disability, medications, cognition, mood, and social support. Reducing the pathophysiological burden of sarcopenia is an underlying element in its treatment (Fig. 6). These guidelines are expected to increase the clinical uptake of sarcopenia by reducing the gap between knowledge and practice and stimulating further active research on sarcopenia diagnosis and management in clinical settings.
Notes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
This study was supported by grants from the Korean Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No. HC20C0157), and the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (No. 2021R1A2C300580111).
AUTHOR CONTRIBUTIONS
Conceptualization: JYB, HWJ; Data curation: JYB, HWJ; Funding acquisition: HWJ; Investigation: JYB, HWJ, KMK, MK, CYP, KPL, SYL, IYJ, OHJ; Methodology: JYB, HWJ, KMK, MK, CYP, KPL, SYL, IYJ, OHJ; Project administration: JYB, HWJ, KMK, MK, CYP, KPL, SYL, IYJ, OHJ; Supervision: JYB, HWJ, KMK, MK, CYP, KPL, SYL, IYJ, OHJ, JYL; Writing-original draft: JYB, HWJ; Writing-review & editing: JYB, HWJ, KMK, MK, CYP, KPL, SYL, IYJ, OHJ, JYL.