Prevalence of Sarcopenic Obesity in Various Comorbidities, Diagnostic Markers, and Therapeutic Approaches: A Review
Article information
Abstract
The coexistence of sarcopenia and obesity characterizes sarcopenic obesity. In this condition, there is an imbalance between lean and fat mass amounts. It is a prevalent issue that is gaining prevalence among the elderly population. To evaluate the condition, allied health professionals may employ non-invasive diagnostic techniques, such as gait speed, skeletal muscle mass, and muscle strength. Nevertheless, early diagnosis and treatment of pathology are essential for preventing debilitating effects and providing the highest quality of care. This article reviews the prevalence of sarcopenic obesity in numerous medical conditions, such as cancer, arthritis, postoperative cases, diabetes mellitus, obesity, and metabolic syndrome. In addition, this paper aims to examine the available evidence regarding the prevalence of sarcopenic obesity in other conditions along with their diagnostic markers and therapeutic approaches.
INTRODUCTION
Obesity has become a grave concern in all age groups. The global pandemic of overweight and obesity, colloquially referred to as "globosity," is rapidly becoming a significant public health concern worldwide.1) Obesity and aging exert enormous demands on the global healthcare system; moreover, the global population is rapidly aging. Over 22% of the population will be over 60 years of age in 2050, with approximately 5% over 80 years of age. As society ages, numerous health concerns progressively increase. For instance, >42% of 55-year-olds globally have trouble performing daily tasks, and 85%–90% require medication for type 2 diabetes, hypertension, and cardiovascular disease. Consequently, the risks of falls, hospitalization, loss of mobility, and premature mortality have gradually increased over the past two centuries.2,3)
Obesity is another major cause of physical limitations in older people. Because sarcopenia and obesity impact each other, the combination of the two, termed sarcopenic obesity (SO), has a much worse effect on physical limitations. Moreover, these conditions result in increased burdens on the healthcare system, resulting in higher healthcare costs. Multiple factors contribute to physical limitations, including age-related skeletal muscle atrophy. As people age, impaired skeletal and muscular function, as well as a disease known as sarcopenia, are significant factors.4) The body composition phenotypes of older adults are categorized as normal, sarcopenic, obese, and a combination of sarcopenia and obesity. Sarcopenia is a common problem in older people that affects the distribution of fat and is linked to functional impairment.5) Sarcopenia is a condition in which muscle mass, strength, and physical function decrease with age. Baumgartner6) defined SO as sarcopenia accompanied by increased adipose tissue. In recent years, interest in age-related changes in body composition, such as the gradual loss of muscle mass and increase in fat mass, has increased.7) More than 50 million people worldwide experience sarcopenia, which is characterized by the loss of skeletal muscle mass and functional impairment.8,9) Over 200 million people will be affected by sarcopenia in the next four decades. Obesity, particularly visceral obesity, is a trend noted particularly among the elderly that promotes metabolic conditions such as hypertension, diabetes, and hyperlipidemia and poses a substantial risk for cardiovascular disease.10)
SARCOPENIC OBESITY
The word "sarcopenia" comes from the Greek words "sarx," meaning "flesh," and "penia," meaning "muscle loss." Owing to the lack of a clear description of the disorder, there remains no standardized method for identifying sarcopenia. Sarcopenia was previously believed to affect only older adults; however, significant muscle loss has been observed in patients with chronic illness, inflammation, and starvation.11) The European Working Group Sarcopenia in Older People (EWGSOP) defines sarcopenia as "a condition characterized by gradual and widespread loss of skeletal muscle mass and strength associated with a risk of adverse outcomes, such as physical impairment, poor quality of life, and mortality." Sarcopenia is associated with physical limitations, falls, prolonged hospital stays, infectious and non-infectious conditions, and an increased overall risk of death. Sarcopenia is not restricted to thin or underweight individuals; as all people age, they lose muscle and gain subcutaneous and intramuscular fat, resulting in SO. Compared with obesity or sarcopenia, SO is associated with a lower quality of life and more disability, illness, and death.12)
Obesity due to sarcopenia can be identified using a variety of approaches and thresholds based on body composition. An appendicular skeletal muscle mass index (SMI; muscle mass in kilograms divided by height in squared meters) greater than two standard deviations below the mean of a young comparison group is a commonly used definition for sarcopenia in the investigation of the aging process. Dual-energy X-ray absorptiometry (DXA) measures lean soft tissue (LST) in the limbs to determine appendicular skeletal muscle mass. Because appendicular LST is almost entirely composed of muscles, it is a reliable indicator of muscle mass. Bioelectrical impedance analysis (BIA) is an additional low-cost method for assessing LST.13,14)
Most studies have defined SO based on the presence of both obesity and sarcopenia. Fewer studies have applied the population distribution of the residuals of linear regression models to predict fat-free appendicular mass using independent factors, such as height in meters and fat mass as kg/m2 to classify SO. To detect SO, two studies evaluated the fat mass to fat-free mass (FFM) or visceral adipose tissue area-to-thigh muscle area ratios. Moreover, various studies have described sarcopenia in obese people as a loss of muscular strength as manifested by decreased handgrip strength (HGS). However, no study has defined sarcopenia as the presence of decreased muscle strength and mass.15) Several studies have reported a lower rate of sarcopenia among participants using statins; however, scientific evidence is lacking.16)
METHODS OF REVIEW
We conducted a literature search of the MEDLINE, PubMed, and Google Scholar databases to identify relevant articles based on the following search strategy: Sarcopenia OR Sarcopenic Obesity Or Obesity AND (prevalence * OR metabolic syndrome OR osteoarthritis OR cancer * OR rheumatoid arthritis * OR diabetes or cardiometabolic OR gait speed OR walking speed OR handgrip strength OR strength). The terms "skeletal muscle OR body composition" were also used. We used "children" and "interventions" as the search terms. The search expanded as the citations in the papers identified during the initial search led to the discovery of additional potentially relevant publications. Papers detailing effects, interventions, and the development of diagnostic tools were ruled out, and the enhanced search method included only prevalence studies with diagnostic and epidemiological emphases. The full texts of the included studies were obtained. Due to our focus on middle-aged and older men and women, we excluded studies with participants <18 years of age without a separate analysis according to age group and studies with both sexes. We also included participants who were overweight or obese (defined as a body mass index [BMI] of 25–30 kg/m2). The retrieved studies were published between 2015 and 2022.
PREVALENCE OF SARCOPENIC OBESITY
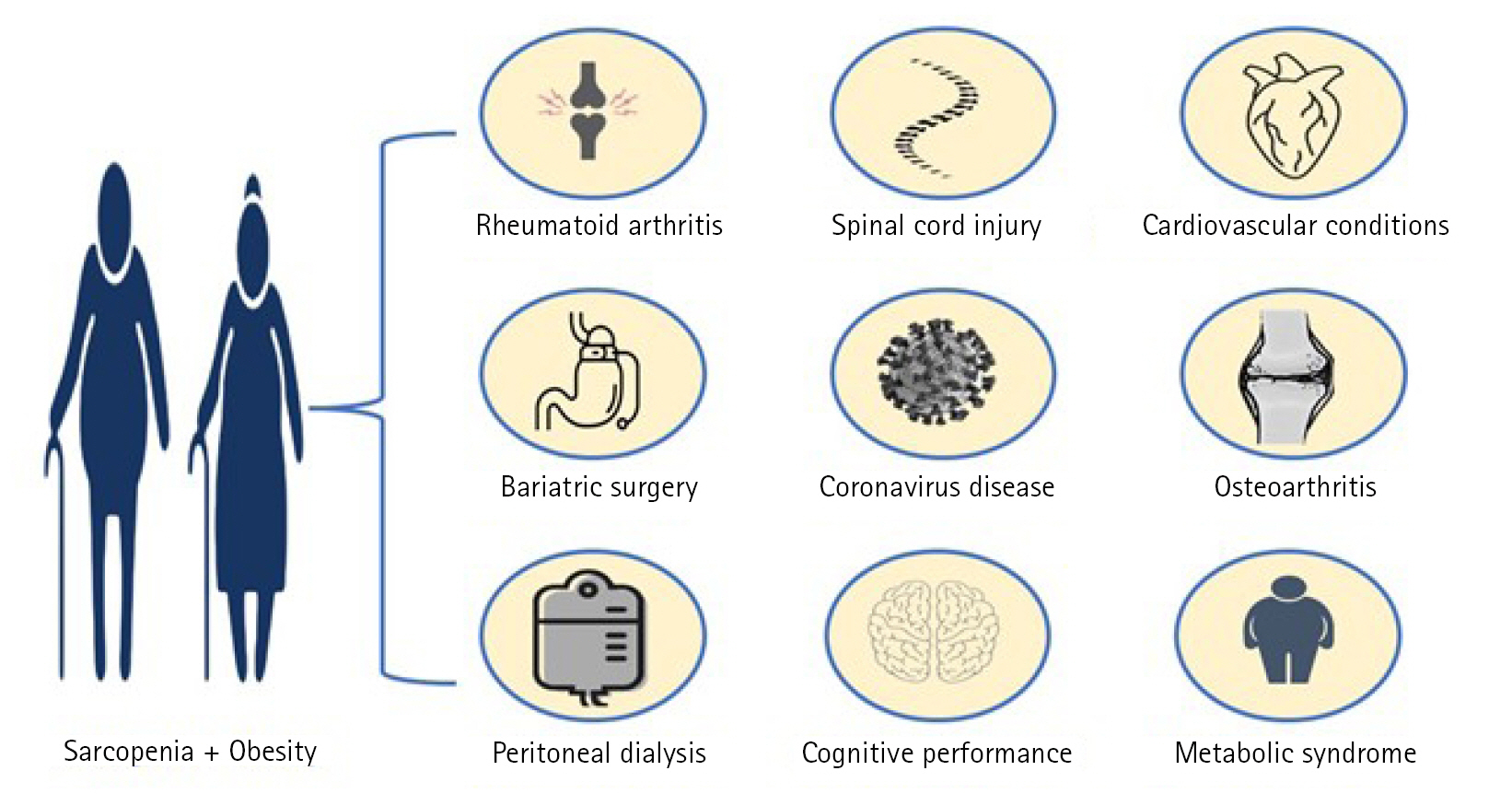
It is difficult to determine the prevalence of SO owing to the absence of a global consensus definition of sarcopenia and the use of different methods for measuring body composition. However, the sarcopenic obese phenotype has been identified in many medical conditions (Fig. 1), likely due to the rising prevalence of obesity worldwide and intense muscle catabolism.
Sarcopenic obesity in Various Comorbidities
Sarcopenic obesity and diabetes mellitus
A cross-sectional study in Singapore including adults (n=1,235) >45 years of age with type 2 diabetes identified SO in approximately one-fifth of the patients (>20%). Furthermore, the study used BIA device analysis and sexual preference cut-offs for appendicular lean mass (ALM)/height2,17) as recommended by Janssen et al.,18) (10.75 and 6.50 kg/m2 for men and women, respectively). Kreidieh et al.19) assessed the prevalence of SO among overweight and obese women seeking therapy and the association between type 2 diabetes, hypertension, and dyslipidemia. They also assessed the potential correlations between these three conditions. Among the 154 individuals, the incidence of type 2 diabetes and hypertension was considerably greater in 31 individuals who were overweight or obese and satisfied the SO criteria. SO was present in nearly 20% of adult women who were obese and under treatment for their condition.
Sarcopenic obesity and cancer
Hom20) examined the incidence of sarcopenia and SO in non-Latino whites and African Americans with and without colorectal cancer using 128 individuals with colorectal cancer and matched cancer-free controls (n=128). In 2020, Kaledkiewicz et al.21) applied conventional techniques to examine the incidence of SO in a case-control study of 103 postmenopausal women >50 years of age with a previous breast cancer diagnosis. The participants were classified into group 1 (78 patients who had completed cancer treatment and had shown reduced or no symptoms for at least 5 years), group 2 (25 patients with cancer recurrence), and group 3 (the control group of 73 women with no history of breast cancer). The prevalence of SO in groups 1, 2, and 3 was 0%–11.5%, 0%–40%, and 0%–4.1%, respectively.
Sarcopenic obesity and osteoarthritis
A cross-sectional study in Korea by Lee et al.22) that included 2,893 participants >45 years of age with knee osteoarthritis (OA) reported a 3% prevalence of SO participants who were obese. Patients with SO were substantially older and had a lower appendicular skeletal mass, higher whole-body fat mass, and a larger waist measurement compared with those in participants with non-SO. Using DXA, Ji et al.23) studied the incidence of sarcopenia and SO in patients undergoing orthopedic surgeries. Their results showed that the incidence of SO varied from 1.8% to 21.2%. Godziuk et al.24) examined the similarity between the phenotype of SO, defined as low muscle mass and high adiposity, in people with advanced knee OA. Patients with and without SO were identified based on diagnostic criteria, including muscle/fat mass, muscular strength, and physical performance. The body composition was then compared based on DXA, and pain, function, and quality of life outcomes were evaluated. Gait velocity, HGS, 6-minute walking test time, pain, physical performance, and health-related quality of life were assessed using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and EuroQol Foundation questionnaire. Among 151 total participants, 59% were women and the average BMI was 37.1±5.5 kg/m2. Among adults, the frequency of SO varied between 1.3%, 14.6%, and 27.2% respectively.24)
Sarcopenic obesity and cognitive function
Obesity and sarcopenia are detrimental to health, especially cognitive function. Moreover, the combination of obesity and sarcopenia may be more dangerous than either condition alone. Tolea et al.25) administered the Montreal Cognitive Assessment (MoCA) test to 353 adults >40 years of age as part of a cross-sectional study to assess the potential link between SO and performance on global and subdomain-specific cognitive assessments. The lowest global cognition score was linked to a type of fat called sarcopenic fat. A cross-sectional study by Yigit et al.26) on 201 participants aged >65 years in Istanbul reported significantly higher scores in older individuals with reduced cognitive function compared to the scores in participants with normal cognitive functions.
Sarcopenic obesity and coronavirus disease
Coronavirus disease 2019 (COVID-19) is a potentially fatal condition caused by infection by the coronavirus responsible for severe acute respiratory syndrome (SARS-CoV-2). Wilkinson et al.27) evaluated the link between sarcopenic status and the potential for severe COVID-19 resulting in hospital admission or death in the UK Biobank among 490,301 individuals aged 37–73 years from the collected general-public data between March 2006 and December 2010. The authors reported that individuals with SO were twice as likely to develop severe COVID-19. Furthermore, SO appeared to increase the risk of severe COVID-19 more than the risk of obesity alone.27)
Sarcopenic obesity, obesity in both sexes, and sarcopenic obesity in postmenopausal women
Johnson Stoklossa28) examined the prevalence of SO among 120 individuals with class II/III obesity. The prevalence of SO varied widely according to the criterion used, with estimates ranging from 0% in women to 84.5% in men. Park et al.29) conducted a cross-sectional study of 2,810 postmenopausal women who had participated in the Korea National Health and Nutrition Examination Survey from 2008 to 2011. The authors identified SO in 14.8% of postmenopausal women. SO was also linked to platelet counts around the maximum healthy range in postmenopausal women.
Sarcopenic obesity and cardiovascular conditions
Chung et al.30) examined the association between sarcopenia and coronary atherosclerosis based on the presence or absence of fat in a group of participants undergoing health checkups. The prevalence of elevated coronary artery calcification (CAC) was 21% among the 1,282 participants (mean age 58.1 years; 75.5% men). When the study population was classified into four groups based on obesity and sarcopenia status, the prevalence of increased CAC was more significant in the SO group compared with non-sarcopenic, non-obese group (40.7%, p<0.001). SO is highly associated with CAC, independent of other risk factors for coronary artery disease. As the world's population ages, heart failure with a stable ejection fraction has become more common. However, the relationship between sarcopenia, reduced muscle mass, and left ventricular diastolic dysfunction remains unclear. Yoo et al.31) assessed the connection between diastolic dysfunction and factors such as sarcopenia and obesity in a cross-sectional study including a large population of 31,258 people at the Health Promotion Center of the Samsung Medical Center in Seoul, Korea. The authors observed diastolic dysfunction in 3,058 (9.78%) participants. Diastolic dysfunction was significantly more common in SO—odds ratio [OR]=1.70; 95% confidence interval [CI], 1.44–1.99. Yang et al.32) reported SO in 7.23% of 844 seniors aged 65 years and older (448 men and 396 women). In addition, obesity and sarcopenia were connected. For example, higher serum high-sensitivity C-reactive protein (hs-CRP) levels were associated with obesity in men who were obese.
Sarcopenic obesity and rheumatoid arthritis
In 2021, Baker et al.33) evaluated the prevalence of SO among three rheumatoid arthritis (RA) cohorts. SO was observed in 12.6% of the patients. Compared to the general public, RA patients showed lower lean mass and increased incidence of SO.
Sarcopenic obesity and metabolic syndrome
SO is characterized by a high percentage of fat and a low percentage of fat-free weight in the body, which contributes to an increased risk of cardiometabolic illness. In their 2021 study, Siervo et al.34) reported the incidence of SO and the link between SO and the risk of metabolic syndrome in 4,296 people in Iran aged 35–70 years. Body composition indices, such as fat mass, FFM, ALM, and SMI were measured and analyzed using BIA. The SO fraction varied from 4% to 26% depending on the method of classification used. Metabolic syndrome was diagnosed in 12.8% of men and 31.6% of women. In addition, a correlation between SO and an increased risk of developing metabolic syndrome has been reported. Poggiogalle et al.35) conducted a study on 727 Caucasian adults aged 18–65 years to determine the prevalence of SO. In this study, 1.0% of men (34.8%) and 0.6% of women had SO (50.1%). Patients with SO had a higher risk of developing metabolic syndrome than those without sarcopenia (47.6%). Metabolic syndrome and low-grade inflammation were also associated with SO in Caucasians. Therefore, patients with SO should consider undergoing metabolic profiling.
Sarcopenic obesity and community-dwelling older adults
A cross-sectional study of 423 individuals over 65 years of age by Ozturk et al.36) investigated the relationship between body composition changes, particularly their clinical components, and quality-of-life factors. Sarcopenic patients with obesity comprised 11% of the study population. Sarcopenia was correlated with adverse health outcomes such as an increased risk of falling and diminished health-related quality of life. A 2018 study in Turkey by Bahat et al.8) to determine the fat percentage cut-off values for obesity and sarcopenic obesity in community-dwelling older adults recruited 992 individuals aged >65 years (308 men and 684 women). The authors observed sarcopenia in 3.1% of the population compared with 0.4%, with SO identified in 0.3% compared to 0.1%. After adjusting the skeletal muscle mass for the square of the height to detect low muscle mass, the low incidence was attributed to an underestimation of sarcopenia among participants with obesity. Lee and Park37) investigated the prevalence of SO and its contributing factors in a cohort study of 338 community-dwelling women >65 years in South Korea. Based on the Asian Working Group on Sarcopenia (AWGS) definition of SO as the upper two quintiles of percent body fat, the prevalence of sarcopenia was 6.2%. In 2015, Kemmler et al.38) conducted a similar study on 1,325 German women aged over 70 years. The cohort showed similar distributions of height, weight, family, education, socio-economic status, lifestyle, and number and distribution of illnesses, and compared to those in the German population. For example, 4.5% of individuals had sarcopenia according to EWGSOP standards, compared with 3.3% of individuals according to the International Working Group on Sarcopenia (IWGS) standards. Kemmler et al.39) reported the prevalence of sarcopenia and SO in a cohort of 965 community-dwelling German men aged >70 years using different sarcopenia (EWGSOP, IWGS, and FINH) and obesity classifications (i.e., BMI and body fat). The incidence of SO varied from 4.1% (EWGSOP + body fat >25%) to 2.1% (IWGS + body fat >30%) across the cohort. Asare et al.40) examined the prevalence of SO among 300 African Americans aged 55–74 years in the United States. According to US Census Centers of Disease Control and Prevention (CDC) data from 84,838 individuals between 2006 and 2008, African American men and women comprised 32.6% (95% CI, 31.4–33.9) and 40.6% (95% CI, 39.7–41.5) of the population in the South. In that study, the proportion of individuals with SO was significantly higher among women.
In 2019, Du et al.41) conducted a study in East China to evaluate sex differences in the incidence and adverse effects of sarcopenia and SO among 213 men and 418 women aged 65 years and older. In this study, 7.0% of men and 2.4% of women had SO. Based on the AWGS criteria, men had a significantly increased risk of sarcopenia and SO compared with that in women. In addition, men with SO had an increased risk of osteoporosis and dyslipidemia; however, women with SO had an increased risk of higher blood glucose levels. A 2017 study by Santos et al.42) reported the prevalence of SO among 1,373 adults aged 65 years and older in the community as well as socio-demographic health problems and problems with functionality and performance related to SO according to the database maintained by the FIBRA Network at the Federal University of Minas Gerais. SO was detected in 4.44% of older adults. Furthermore, SO was related to insufficiency in complex and fundamental daily activities and gait speed and also significantly increased the likelihood of pre-frailty and frailty. A 2020 study by de Campos et al.43) examined 270 older adults to assess the incidence of SO and its association with functionality, lifestyles, biological indicators, and morbidity. The prevalence of SO in Brazil was 29.3%. Scott et al.44) examined whether decreased muscle mass (sarcopenia) or strength (dynapenia) in the presence of obesity was associated with an increased risk of osteoporosis and non-vertebral fracture among 1,089 community-dwelling individuals aged 5–10 years for which baseline measurements were collected. Compared to women without SO, women with sarcopenic obesity had a higher risk of fracture (2.8; range, 1.4–5.6). A 2016 study by Perna et al.45) assessed the incidence of sarcopenia and SO in hospitalized older adult patients and examined their metabolic profiles and significant changes compared to those in healthy controls. Their study included 639 participants >65 years of age (196 men and 443 women). Of the SO population, 8.13% were female and 22.45% were male.
Mo et al.46) investigated the frequency of SO and diagnostic concordance of the condition in 1,050 Chinese community-dwelling older adults, including 347 men (71.3 years of age) and 703 women (69.9 years) using a variety of obesity diagnostic approaches. The incidence of SO ranged from 0.1% to 7.9%. In 2016, Moreira et al.47) utilized BIA to determine the prevalence of SO and the relationship between SO and physical performance in 491 middle-aged women in Northeast Brazil. In their study, 7% of the participants had SO, which was linked to poor physical performance. Du et al.48) reported that the prevalence of sarcopenia and SO differed significantly across racial and ethnic subpopulations of older people. Hispanics had the highest prevalence of sarcopenia and SO, whereas African Americans had the lowest prevalence. Developing effective medical screenings and interventions for an increasingly diverse population of older adults in the United States may require a deeper understanding of these differences. Wagenaar et al.49) analyzed 119 participants of the Dutch Lifelines to determine the prevalence and causes of SO and sarcopenic obesity overweight (SOW) in the general public. Muscle mass was determined based on sex-specific analysis of 24-hour urine creatinine excretion. Obesity was defined as a BMI ≥30 kg/m2, whereas overweight was defined as a BMI ≥25 kg/m2. The prevalence of SO was 0.9% in men and 1.4% in women, whereas the frequencies of SOW were 6.5% and 6%, respectively. The incidence of SOW and SO was greater in women than in men across all age categories, except for men aged 40–59 years, who had a higher incidence of SOW. In addition, age was a significant factor in SO and SOW, with the frequency increasing after 50 years of age. However, 82.5% of individuals with SO or SOW were <70 years of age. Wang et al.50) conducted a cohort study to investigate the association between SO and cognitive impairment in community-dwelling participants aged 60–92 years. SO was detected in 6% of the population (7.3% of men and 4.8% of women). The incidence of SO was relatively low among community-dwelling Chinese individuals >60 years of age overall (6.0%). Von Berens et al.51) also assessed SO in 521 individuals aged ≥75 years who participated in the Gothenburg H70 Birth Cohort Studies and 288 men aged 87 years who participated in the Uppsala Longitudinal Study of Adult Men (ULSAM), in which 4%–11% of senior citizens had SO.
Ishii et al.52) examined the association between depressive symptoms, sarcopenia, and obesity in Japanese seniors. From the resident records of Kashiwa City in Chiba, Japan, 1,731 Japanese people >65 years of age (875 men and 856 women) living independently in the community were randomly selected. Depression affected 10.1% of the population, while 3.7%, 13.6%, and 3.7% were affected by sarcopenia/obesity, sarcopenia or non-obesity, and sarcopenia/non-obesity, respectively. The data suggested that SO has a compounding influence on the risk of developing depressive symptoms, particularly in individuals aged 65–74 years. Kwon et al.53) examined SO in Korean women >60 years of age in good health. Of the 2,396 women aged 65 years and older, 1,491 (62.2%) were underweight, average weight, or overweight, while 905 (37.8%) were obese. Using a cut-off value of 5.4 kg/m2, sarcopenia was identified in 64.9% of underweight women (63/97), 38.2% of normal-weight women (320/838), 17.1% of overweight women (95/556), and 6.1% of obese women (55/905). Lim et al.54) examined the frequency of sarcopenia and SO in older adult Koreans and their relationship with chronic disease. They divided the 3,492 participants into three groups (non-sarcopenia, sarcopenia, and SO) and performed statistical analyses to compare general anthropometry, health behavior, food consumption, and chronic illness status among the groups. Compared to the lean category, the sarcopenic obese cohort ingested considerably more calories (p=0.005), protein (p=0.046), and fat (p=0.001). Furthermore, among the three groups, the cohort with the most significant diabetic population (p=0.023) and dyslipidemia (p=0.004) had sarcopenia and obesity, respectively.
Sarcopenic Obesity in Specific Medical Conditions
Sarcopenic obesity and postsurgical conditions
In their 2016 retrospective study, Carias et al.55) reported factors predicting sarcopenia and SO in 207 patients with cirrhosis undergoing liver transplantation (LT) between January 2008 and December 2013. Men comprised 68% percent of the study population. SO was identified in 41.7% of patients. Nishigori et al.56) examined the association between body composition and surgical site infection (SSI) after total laparoscopic gastrectomy (LTG). A total of 157 patients with gastric cancer who underwent LTG at Kyoto University Hospital between March 2006 and October 2014 were retrospectively studied. SO was detected in 28 of the population (18%). Sarcopenia, similar to LTG, is a risk factor for developing SSI. Gan et al.57) examined the association between cholecystectomy and low muscle mass, poor muscle strength, sarcopenia, and SO in 4,909 people aged 18–80 years. Individuals who underwent cholecystectomy had lower muscle mass, strength, and sarcopenia compared to those who did not. Furthermore, those who underwent cholecystectomy more than 7 years prior were more likely to develop sarcopenia compared to those who had recently undergone cholecystectomy (the average time between cholecystectomy and a physical assessment was 7 years). Cholecystectomy has also been linked to decreased muscle mass, strength, and sarcopenia. In 2019, Anastacio et al.58) reported the physiological changes, prevalence, and associated variables of sarcopenia, obesity, and SO after transplantation in 100 patients (52.6±13.3 years; 57.0% male). The fat mass index decreased from 17.9±2.5 to 17.5±3.5 kg/m2. However, the fat mass increased from 8.5±3.5 to 9.0±4.0 (p<0.05). Although the increase was not statistically significant, the rates of sarcopenia (19.0%–22.0%), obesity (32.0%–37.0%), and SO (0%–2.0%) all increased. Sarcopenia is also more common in women than men.
Sarcopenic obesity and spinal cord injury
In their cross-sectional study, Pelletier et al.59) established the incidence and clinical utility of spinal cord injury (SCI)-specific and general population obesity and SO thresholds in tertiary SCI rehabilitation institutions in Toronto. The participants were 136 patients aged >18 years with chronic SCI for at least 2 years who provided informed consent. Participants who were above the weight limit of the densitometer (123 kg), were pregnant or trying to become pregnant, or with a disease known to influence bone metabolism were excluded. Obesity associated with sarcopenia was observed in 41.9% of the participants. Moreover, SO was common among individuals with long-term SCIs.
Sarcopenic obesity and bariatric surgery
Molero et al.60) investigated the effect of age on the incidence of SO among applicants for a bariatric surgery. A total of 1,370 patients >18 years of age who underwent bariatric surgery were categorized according to age (18–39, 40–49, 50–59, and >60 years). The incidence of SO in class I and II were 16.4%, and 4.6%, respectively. Women showed a higher incidence of SO (p=0.005), and the proportion of women with SO increased with age, whereas no such correlation was observed in men. Class I SO was observed in 29.1% of women >60 years of age compared with 12.8% among those with class II SO. Percent SMM showed comparable results (Cohen's coefficient=0.888, p=0.001). Thus, age was a potential risk factor for SO in patients undergoing bariatric surgery, and SO was prevalent among women bariatric surgery candidates aged ≥60 years.
Sarcopenic obesity and stroke
A cross-sectional study by Matsushita et al.61) included 376 consecutive 65-year-old patients admitted for post-stroke rehabilitation between January 2017 and March 2019 to better understand the relationship between SO, simple obesity, and ADL in patients with stroke in convalescent rehabilitation wards. In that study, 46% (172/376) of the 376 patients were obese (mean age, 77.7 years; 117 men and 55 women), while 28% (107/376) were classified as having SO. The patients with SO were significantly older (p=0.001), had a higher BMI (p=0.001), and had a significantly higher percentage of body fat compared to those with simple obesity (p=0.001).
Sarcopenic obesity and peritoneal dialysis
Tabibi et al.62) reported the prevalence of SO in Tehran in 2018 and its association with cardiovascular disease (CVD) risk factors in patients with Parkinson disease. SO was identified in 3.8% of patients with Parkinson disease. These patients have significantly elevated levels of hs-CRP, soluble intercellular adhesion molecule type 1, triglycerides, total cholesterol, and low-density lipoprotein cholesterol. In addition, the blood concentrations of CVD risk factor markers were more remarkable in patients with Parkinson disease and SO compared with those in obese individuals without SO. These findings suggested that Parkinson disease and sarcopenia were associated with CVD risk factors, despite the low prevalence of SO in patients with Parkinson disease. Malhotra et al.63) investigated the influence of different definitions on the incidence of SO in terms of mortality in a database of patients undergoing hemodialysis. The average age of the 122 participants was 46 years (interquartile range, 40–54 years) for men and 50 years (44–61 years) for women. SO was identified in 12% to 62% of men and 2% to 74% of women.
Diagnostic Markers of Sarcopenic Obesity
Aging, inactivity, poor diet, and lifestyle variables including insulin resistance (IR), systemic inflammation, and oxidative stress play roles in SO development and lead to decreased total muscle mass, muscle quality, and muscle strength, in addition to increased fat mass. Increased adipocyte size and proliferation, and immune cell infiltration of adipose tissue, trigger an inflammatory reaction. Adipocytes produce adipokines and immunological cells, an inflammatory reaction that is not confined to the fat tissue but rather extends throughout the body.64) Mitochondrial malfunction and unbalanced myokine production by myocytes—including myostatin, irisin, tumor necrosis factor-alpha (TNF-α), and interleukins (ILs)—may occur due to inflammatory processes and fat buildup. These could be potential biomarkers of SO. Some studies have proposed the use of serum myostatin as a predictive marker for the early diagnosis of sarcopenia and other forms of muscle wasting associated with aging.65,66) One study reported higher serum insulin-like growth factor 1 (IGF-1) levels in older adults with SO who performed weight training compared to those in the SO group that did not perform weight training. These findings provided evidence that a deficit in IGF-1 increased the risk of developing SO.67,68) Several studies have reported the potential correlation between fibroblast growth factor 21 (FGF21) disruption and sarcopenia and SO. However, the influence of FGF21 on either of these illnesses remains unclear; thus, further in-depth research on the function of FGF21 as a diagnostic marker for SO is required.69-71) The inflammatory factors IL-6 and TNF-α are upregulated in SO in older adults.72) Another study reported the association between elevated IL-6 levels and SO in an aged population.5) Moreover, low plasma levels of IL-15 were associated with sarcopenia development.73) The usefulness of IL-15 as a biomarker requires further research in both scientific and therapeutic contexts. Other myokines, including leukemia inhibitory factor (LIF), angiopoietin-like 4 (ANGPTL4), apelin, β-aminoisobutyric acid (BAIBA), decorin, meteorin-like (Metrnl), myonectin, and secreted protein acidic and rich in cysteine (SPARC), are also potential tools for predicting the development of SO74); however, the evidence is reported in a limited number of studies.
Therapeutic Approaches for Sarcopenic Obesity
Individuals with severe conditions cannot perform heavy workouts; therefore, blood flow occlusion training has recently been advised.75) Several studies have shown that low-intensity blood flow occlusion may build muscle mass and strength as well as the capability for high-intensity strength training. Thus, exercise therapies and blood flow restriction may be valuable methods to avoid worsening sarcopenia and obesity.75) Independent of the use of other amino acids, leucine supplementation in older individuals was associated with increased muscle protein synthesis rates.76) Studies are underway to determine the efficiency of dietary interventions in avoiding muscle atrophy in older adults. Even as changes in the way of living are an essential component of the treatment of SO, these modifications may not be possible in some instances due to limits imposed by the body or a lack of adherence. Other breakthrough treatment options also exist for patients with SO. The decrease in testosterone due to aging occurs concurrently with the age-related reduction in lean body mass and increase in body fat, causing SO.77) Testosterone replacement therapy in hypogonadal males improves body composition and muscular strength.78) The function of myostatin in SO has recently attracted attention, with data suggesting that blocking myostatin could lead to beneficial alterations in fat and muscle composition. Participants in phase I or phase II clinical studies of a myostatin antibody for the treatment of muscular dystrophy did not show improved muscular endurance or function, although the study was not designed to assess the treatment effectiveness.79) One study reported that inhibiting myostatin in individuals with muscular dystrophy improved cellular-level muscular activity but did not contribute to measurable increases in muscle endurance.80) Inhibiting myostatin could reduce the risk or management of SO; however, long-term studies are needed to understand this relationship, with studies underway in healthy individuals. The dietary form of anamorelin stimulates appetite in individuals with cancer cachexia and shows potential for treating patients with SO. The anti-inflammatory and anabolic qualities of anamorelin may help counteract the unfavorable nitrogen balance that is characteristic of sarcopenia.81)
DISCUSSION
To our knowledge, this study is the first to discuss the prevalence of SO in the presence of various comorbid conditions. In this study, based on the criteria and study samples, the estimated incidence of SO in older adults ranged from 0.9% to 81.1%, with a significantly higher prevalence among those aged 65 years and older. Most research has been conducted on community-dwelling individuals. However, sarcopenia has emerged as a significant public health concern because of both the rapidly increasing older population and widespread obesity, which has been associated with detrimental cardiometabolic effects and adverse health outcomes.82) One in six individuals worldwide will be 60 or older by 2030, according to the World Health Organization.83) By that time, there will be 1.4 billion people over the age of 60 years, up from 1 billion in 2020. The number of persons in the globe who are 60 years or older will double by 2050. (2.1 billion). Between 2020 and 2050, the number of people 80 years or older is projected to treble, reaching 426 million. In 2047, senior citizens are projected to outnumber children for the first time in recorded history. Based on previous estimates of SO prevalence and the global population of older adults, SO currently affects 40–80 million individuals, which will increase to 100–200 million individuals within the next 35 years. This group is newly considered in the study of sarcopenia and SO, and research in this field is still in its infancy.83,84) Estimates of SO prevalence have fluctuated widely in the past, depending on the sarcopenia diagnostic criteria, muscle mass assessment methods, research settings, and geographic locations. Therefore, no variations in prevalence could be considered statistically significant among the various diagnostic criteria for obesity. Moreover, aging modifies body fat distribution, resulting in subcutaneous fat loss, visceral fat gain, and ectopic fat deposition. Multiple metabolic processes contribute to aging-associated SO. Basal metabolic rate and metabolic adaptations such as adaptive thermogenesis decline with age, resulting in decreased muscle mass and increased body fat. Aging also leads to decreased resting metabolic rate, physical fitness, mitochondrial volume, and oxidative capability, all of which contribute to decreased muscle size and strength.84)
The results of the present study also showed no statistically significant differences in the incidence of SO according to sex, indicating that both men and women are at risk. This could be due to the decreased sex-specific hormone levels in older adults. SO is significantly affected by hormonal differences between men and women. After menopause, women experience increased body weight and fat mass as well as a shift from subcutaneous to visceral fat storage.85) In addition, lower testosterone levels are associated with sarcopenia, decreased muscular strength, poor physical performance, and an increased risk of falling in older men whose total testosterone levels decline by approximately 1% per year.86)
Muscle mass and strength begin to decrease around 30 years of age, with a sharp increase in the rate of decline after 60 years of age. In contrast, the amount of body fat increases steadily with age, peaking between 60 and 75 years, and this can lead to obesity. Thus, older adults may be more susceptible to SO owing to these physiological factors.87) In addition, the pooled prevalence of SO was higher in hospitalized patients than in community residents in the included studies. This may be because malnutrition and a sedentary lifestyle are linked to SO incidence; thus, hospitalized patients may experience malnutrition due to disease or other therapeutic strategies; moreover, complete bed rest leads to low physical activity levels. Based on the results of pooled-effects analyses, several studies reported greater mortality risk among hospitalized older adults with SO compared with that in older individuals living in the community.88)
However, no drugs are licensed for the treatment of SO because its pathophysiology is still unclear; thus, lifestyle modifications, especially exercise and dietary interventions, are the most common care recommended.89) Resistance training, aerobic exercise, and full-body electrical muscle stimulation are the most prescribed physical activities. Nutritional therapies include supplementation (with protein, amino acids, or vitamin D) and dietary changes to improve health.90-92) These therapies may be prescribed independently or in conjunction. As discussed previously, systematic studies have demonstrated the effectiveness of therapies focusing on exercise and diet for the treatment of SO. However, multi-center studies with large sample sizes are needed to further study SO in hospitalized older adults. Doctors should prioritize SO screening for future clinical procedures.
In conclusion, obesity and aging are the two significant health risks for adults worldwide. Both factors increase the likelihood of illness or other health problems. SO occurs when both sarcopenia and obesity are present in the body and significantly impact the health of older adults. Many effective strategies have been developed to investigate the prevalence of SO. The present study focused on the increasing incidence of various conditions because SO has a multiplicatively deleterious impact on physical performance and general health. The present review aimed to summarize the occurrence rates of different disease conditions. In addition, the comparison of research efficacy was complicated because SO is defined in various ways. However, the results of the present study demonstrated that SO is multifactorial, necessitating multi-targeted treatment. Thus, screening for SO in older adults should be a priority to allow the early identification of at-risk individuals and the implementation of suitable treatments to minimize the incidence of unfavorable outcomes and improve quality of life and healthy aging. Thorough physical examinations are essential for the correct diagnosis of SO. The top scientific priorities are evaluating SO and the comprehensive testing of therapeutic alternatives via randomized controlled trials. Awareness of SO is also essential, which may help to determine the exact proportion of SO worldwide under different conditions. These findings support the argument for a broader focus on SO beyond geriatrics, particularly among individuals with multiple medical conditions.
Notes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization, MK, AK; Data curation, MK, AK; Funding acquisition, NA; Investigation, MK, AK; Methodology, MK; Project administration, MK, AK; Supervision, AK; Writing-original draft, MK ; Writing-review & editing, AK.