Hyperglycemic Hemichorea: A Case Series
Article information
Abstract
Hemichorea has been reported in subjects with hyperglycemia and the recurrent was rarely reported. We identified clinical presentation and course of hypeglycemic hemichorea in six cases, one of whom experienced recurrence. Mean age was 77.2 years and five were women. Duration of diabetes was 1-35 years. Initial HbA1c levels were 9.3%-13% and most patients had poor compliance. Five patients undertook brain magnetic resonance imaging and high signal intensities of basal ganglia was observed in T1-weighted images. All patients were treated with insulin, and four patients were treated with dopamine-receptor blockers. Mean duration of hemichorea was 69 days. One patient’s hemichorea was recurred after 2 months from the time when the first event was resolved and then finally diagnosed with vasculitis. The prognosis of hyperglycaemic hemichoerea seemed to be good. However, we should consider other cause such as vasculitis when the symptom was recurred after adequate glycaemic control.
INTRODUCTION
Hemiballism and hemichorea are rare hyperkinetic movement disorders that are characterized by irregular unilateral flinging or throwing movements of the limbs1) and involve the contralateral basal ganglia.2) These disorders can be caused by various medical conditions such as cerebrovascular diseases, infections, tumors, drugs, and metabolic and immunological disorders.3)
Hyperglycemic hemichorea frequently occurs in older patients with poorly controlled type 2 diabetes mellitus (T2DM) and occasionally in those with type 1 diabetes mellitus.4) In most cases, chorea improves gradually over several days after achieving stable glycemic control, although some cases can last for months or even years.5)
This report describes hyperglycemic hemichorea occurring in six older patients with poorly controlled T2DM, one of whom experienced recurrence.
CASE REPORT
We identified six cases of hyperglycemic hemichorea from 2017 to 2020 in our tertiary hospital (Table 1). The mean age of the patients was 77.2 years (range, 67–84 years), and five patients were women. The duration of diabetes varied (range, 1–35 years); however, initial glycated hemoglobin (HbA1c) levels at the patients’ first visit were consistently high (range, 9.3%–13%). The patients had multiple comorbidities such as hypertension (n=4), dyslipidemia (n=4), and chronic kidney disease (n=4). Three patients were treated with insulin, and most patients had poor compliance with medication and irregular visits to the outpatient clinic. This is a retrospective observational study, and we could not obtain written informed consents.
Brain T1-weighted magnetic resonance imaging (MRI) showed high signal intensities in the basal ganglia on the contralateral side to the disordered movements in four of five patients (Fig. 1A, 1B). One patient underwent 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) for the differential diagnosis of bilateral abnormalities observed on brain MRI, which showed bilateral hypometabolism in the corresponding lesions (Fig. 1C).
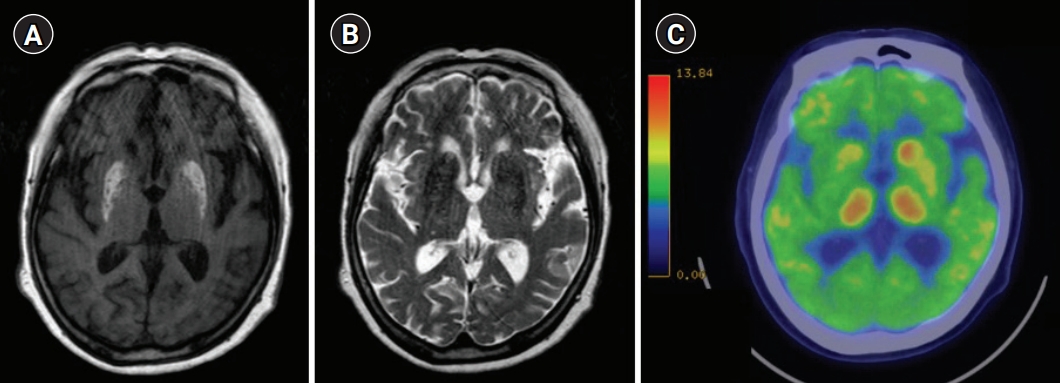
Findings of brain magnetic resonance imaging and brain 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) at the first event in Patient no# 2. (A) High signal intensities in the bilateral basal ganglia on T1-weighted imaging. (B) Low signal intensities in the bilateral basal ganglia on T2-weighted imaging. (C) Brain 18F-FDG PET image showing hypometabolism in the bilateral ganglia, especially in the putamen.
All patients were treated with insulin, with the initial treatment comprising multiple daily insulin injections (total daily dose, 18–36 IU). Two patients were administered sliding-scale insulin injections during their hospitalizations in the emergency and neurology departments and were switched to oral hypoglycemic agents after discharge. Dopamine receptor blockers and benzodiazepines were recommended for four patients. The patients’ symptoms improved gradually after reducing their hyperglycemia, which was stabilized within 3–6 months after the event (Table 1). We could not follow-up on the HbA1c level of one patient who was transferred to another hospital after resolving chorea. The mean duration of hemichorea was 69 days (range, 10–172 days). One patient experienced chorea recurrence 2 months after the first event had resolved. At the time of recurrence, the patient’s glucose level was not aggravated (70–200 mg/dL). The newly developed signs were fever and skin rash without evidence of infection. The patient underwent skin biopsy and laboratory tests and was finally diagnosed with vasculitis. She was transferred to the rheumatology department and treated with immunosuppressants.
DISCUSSION
This report described the clinical presentations and courses of hemichorea in six older patients with T2DM. Their symptoms resolved after achieving adequate glycemic control with insulin and oral hypoglycemic agents. Hemichorea recurred 2 months after the resolution of the initial event in one of the six patients, who was finally diagnosed with vasculitis.
Bedwell6) first described hyperglycemic hemichorea in 1960. The typical pattern of hyperglycemia-induced chorea is unilateral involuntary movement with abnormal neuroimaging in the contralateral basal gangli.7) However, bilateral involvement appeared as generalized chorea in 11.4% of 54 cases.8) Hyperglycemic hemichorea is more common in women, and older age is a known risk factor.8) The findings in our cases are consistent with those of previous reports and have important clinical implications given the increasing numbers of older patients with diabetes. If patients present with involuntary movement, their glycemic status should be evaluated, and brain MRI should be performed to diagnose hyperglycemic hemichorea. The typical findings on brain MRI of patients with hyperglycemic hemichorea include high signal intensity on T1-weighted images and various signals in T2-weighted images of the basal ganglia and putamen on the side contralateral to the disordered movements.9) Negative findings on brain MRI have rarely been reported,10) which we also observed in one of our patients. Brain computed tomography may show hyperdensity in the same lesion.9)
The suggested pathogenesis of hyperglycemia-related hemichorea involves petechial hemorrhage1) or cerebral ischemia with the depletion of gamma-aminobutyric acid and acetylcholine in the basal ganglia, which results in involuntary movements.11) More directly, FDG PET shows a marked reduction in regional cerebral glucose metabolism in the basal ganglia on the affected side, which provides evidence of the disorder or failure of cerebral glucose metabolism in the brain.12) We confirmed this phenomenon using 18F-FDG PET in our patient. To our knowledge, this is the first report of hyperglycemic hemichorea related to bilateral lesions on 18F-FDG PET.
The most important treatment for hyperglycemic hemichorea is the correction of hyperglycemia.5) Hemichorea improves over days to weeks after achieving glycemic control alone in most cases; however, additional symptomatic therapy should be considered if symptoms persist or become severe.5) Such therapy can include dopamine receptor blockers such as haloperidol,8) benzodiazepine, carbamazepine, or valproate.5) Recurrence has been reported in a small number of patients, in whom recurrent hyperglycemia or discontinuation of dopamine receptor blockers may be involved.13) However, the prognosis is generally good, and in our opinion, bilateral presentation might be related to other causes of chorea and poor prognosis.
In conclusion, the prognosis of hyperglycemic hemichorea appears to be good. This condition can be detected based on its typical radiological findings on brain MRI and 18F-FDG PET. However, other possible causes of abnormal movement such as vasculitis should be considered in cases of bilateral involvement and recurrence after achieving glycemic control.
Notes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization, SM, HEK, TJO; Data curation, SM, HEK; Investigation, SM, HEK, TJO; Methodology, SM, TJO, CHA; Project administration, SM, HEK; Supervision, TJO, SHC, HCJ; Writing-original draft, SM, TJO; Writing-review & editing SM, HEK, TJO, CHA, SHC, HCJ.

