|
 |
- Search
| Ann Geriatr Med Res > Volume 28(1); 2024 > Article |
|
Abstract
Background
This study investigated the prevalence of adrenal insufficiency among patients admitted for total knee arthroplasty (TKA) due to osteoarthritis and identified factors contributing to adrenal insufficiency.
Methods
We divided the patients into two groups based on the results of preoperative standard-dose short synchronous stimulation tests: group 1 (adrenal insufficiency) and group 2 (normal adrenal function). We also assessed the prevalence of adrenal insufficiency and compared the numbers of patients who received oral steroids, the frequency of previous steroid injection use, and the frequency of systemic symptoms of steroid depletion such as fatigue and loss of appetite between the two groups. Multiple regression analysis was performed to identify factors related to adrenal insufficiency.
Results
The prevalence of adrenal insufficiency was 60.0% (120/200). Group 1 had higher numbers of previous steroid injections (12.8±10.2 vs. 6.8±7.9) and patients taking oral steroids (18/120 vs. 3/80) (p<0.001 and p=0.011, respectively). The frequency of systemic symptoms of steroid depletion, such as fatigue and loss of appetite, was also higher in group 1 (94/120 vs. 42/80, p<0.001). Recent steroid injections and loss of appetite were associated with adrenal insufficiency (p=0.002 and p=0.009, respectively).
Conclusion
The results of this study revealed a high prevalence of adrenal insufficiency in Korean patients hospitalized for TKA due to end-stage osteoarthritis. Recent steroid injections were causally related to the development of adrenal insufficiency. Therefore, adrenal function should be assessed preoperatively to prevent postoperative complications related to adrenal insufficiency.
The prevalence of adrenal insufficiency is approximately 250–400 per million, while that of secondary adrenal insufficiency is 150–280 per million.1,2) Individuals with advanced osteoarthritis may be at risk of developing adrenal insufficiency because of repeated oral steroid administration or intra-articular steroid injections.3,4) Individuals with adrenal insufficiency may be able to perform daily activities without showing any symptoms.5) However, once they are exposed to stressful situations, such as infection, trauma, or surgery, they may experience life-threatening adrenal crisis.6) The symptoms of adrenal crisis include weakness, nausea, vomiting, and abdominal pain. In surgical patients, it is often difficult to differentiate these symptoms from those of cerebrovascular accidents, ileus, and sepsis.7) However, untreated adrenal crisis can develop into coma and hypotension, with a high mortality rate.7,8) As symptoms of adrenal crisis are nonspecific, early diagnosis is difficult unless the underlying adrenal insufficiency is identified in advance.
In particular, older adult patients who undergo total knee arthroplasty (TKA) for severe osteoarthritis are more difficult to manage than other age groups in terms of the incidence rate, symptom ambiguity, and mortality rate.9,10) As a result, these patients may be misdiagnosed, and the optimal timing of treatment may be missed. To promptly diagnose adrenal crisis and provide timely and appropriate management, patients must be assessed for adrenal insufficiency before surgery and patients with insufficiency carefully monitored for adrenal crisis symptoms after surgery. However, because the prevalence of adrenal insufficiency is very low, the evaluation of adrenal function before surgery is not routinely performed in practice.
The present study investigated the prevalence of adrenal insufficiency among patients in Korea admitted for TKA who underwent assessment using a standard-dose short synacthen stimulation test and identified the contributing factors associated with adrenal insufficiency.
This prospective cross-sectional study was conducted from March to December 2022 after receiving the approval of the Institutional Review Board of our institution. We recruited all patients hospitalized for TKA for severe arthritis (Kellgren–Lawrence grade 3 or 4).11) We obtained informed consent from all patients before their study inclusion. The exclusion criteria were rheumatoid arthritis, osteonecrosis, neuropathic arthropathy, and revision total TKA. Patients who refused to participate were also excluded (Fig. 1).
We surveyed the patients for a history of steroid use as a treatment modality, such as spinal nerve block, intra-articular injection, autoimmune connective tissue diseases, asthma, chronic obstructive pulmonary disease, skin diseases, and a family history of autoimmune diseases. We determined the number of steroid injections in the past 3 months using a questionnaire. We reviewed the medical records of the relevant medical institutions for patients who had received so many steroid injections that they could not accurately recall the number of times they had received them. We also investigated the history of oral steroid administration and daily dose of oral steroids in the past 3 months.
Before hospitalization, we asked the patients about symptoms such as fatigue and loss of appetite that might appear during steroid depletion.12,13) Fatigue was defined as an average score of ≥4 on the 11-point rating scale in the brief fatigue inventory.14) We assessed appetite using visual analog scale (VAS) ratings (0–100 mm; 0 = no appetite at all, 100 = very good appetite).15,16) Loss of appetite was defined as a VAS score for appetite of ≤70.15) We also assessed whether the patients had been previously diagnosed with adrenal insufficiency. Patients who were taking hydrocortisone discontinued it 24 hours before the short synacthen stimulation test, and those taking prednisone or prednisolone switched to an equivalent dose of hydrocortisone 1–2 weeks before the stimulation test and discontinued it 24 hours before.
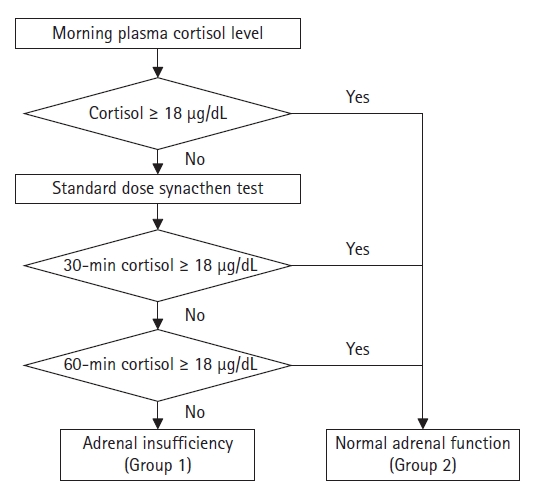
On the morning of the day of surgery, the patients underwent a standard-dose short synacthen stimulation test, in which 250 µg of synacthen was intravenously administered immediately after taking a blood sample to measure the basal blood cortisol concentration.17) We measured blood cortisol concentrations before and 30 and 60 minutes after synacthen administration. A normal basal blood cortisol concentration of ≥18 µg/dL was defined as normal adrenal function regardless of the results of the synacthen stimulation test. Adrenal insufficiency was defined as a blood cortisol concentration of <18 µg/dL at 30 and 60 minutes after synacthen administration. Patients diagnosed with adrenal insufficiency were classified into group 1, while those with normal adrenal function were included in group 2 (Fig. 2). An endocrinologist verified the diagnostic protocol for adrenal insufficiency.
We also assessed the prevalence of adrenal insufficiency. We compared the proportions of patients who had received steroid injections within the past 3 months and the number of steroid injections between the two groups. Similarly, we also compared the proportions of subjects who had taken oral steroids daily in the last 3 months. The correlation between the prevalence of adrenal insufficiency and the American Society of Anesthesiologists (ASA) classification18) was analyzed. Finally, we compared the frequency of the manifestation of symptoms of steroid depletion between the two groups.
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Means and standard deviations are used to describe the data. Comparisons between the two groups were conducted using the Student t-test for continuous variables and the chi-square test for categorical variables. We performed a multiple logistic regression analysis to identify factors associated with adrenal insufficiency among the variables that showed statistically significant differences between the two groups. Statistical significance was set at p<0.05.
Primary approval was obtained from the Institutional Review Board of Kangwon National University Hospital (No. KNUH-2022-01-021-001). Informed consent was obtained from all the study participants. This study complied the ethical guidelines for authorship and publishing in the Annals of Geriatric Medicine and Research.19)
Among 226 recruited patients, 200 were enrolled in the study. Their demographics according to the groups are presented in Table 1. Forty-seven patients were male and 153 were female. The mean age was 72.8±6.9 years. The mean body mass index was 27.0±3.6 kg/m2. Of 200 patients, 120 (60.0%) were diagnosed with adrenal insufficiency. None of the patients had been previously diagnosed with adrenal insufficiency. One hundred and one patients underwent unilateral TKA, and 99 underwent simultaneous or sequential bilateral TKA. There was no significant difference in the prevalence of adrenal insufficiency according to the bilaterality of the joint arthroplasty (p=0.299). Two patients in group 2 had preoperative serum sodium levels <135 mmol/L (122 mmol/L and 132 mmol/L, respectively). No patients in group 1 had a preoperative serum sodium level <135 mmol/L. The distribution of ASA classifications did not differ significantly between the two groups (p=0.491). The basal plasma adrenocorticotropic hormone (ACTH) and basal and stimulated blood cortisol levels are shown in Table 2.
The mean numbers of steroid injections were 12.8±10.2 in group 1 and 6.8±7.9 in group 2 (p<0.001). In the past 3 months, 77.5% (93 of 120) patients in group 1 and 55.0% (44 of 80) patients in group 2 had received steroid injections, and 14.2% (17 of 120) patients in group 1 and 5.0% (4 of 80) patients in group 2 had taken oral steroids (p<0.001 and p=0.038, respectively) (Table 3). However, of the 62 patients with no history of injection or oral steroid use within 3 months before surgery, 26 (41.9%) were diagnosed with adrenal insufficiency.
The principal findings of this study were as follows: (1) the overall prevalence of adrenal insufficiency was 60.0% in patients hospitalized to undergo primary TKA for osteoarthritis, and (2) the frequency of steroid injections administered within 3 months before surgery and the loss of appetite, which is one of the symptoms of steroid depletion, were predictive factors of adrenal insufficiency.
Adrenal insufficiency is a rare disease affecting only 2–4 people per 10,000 people in the population.1,2) Individuals who have not experienced serious stress may continue their daily lives without any significant symptoms. Owing to its low incidence and lack of symptoms under normal circumstances, routine preoperative screening for adrenal function is typically not performed in patients undergoing orthopedic surgery. However, patients with adrenal insufficiency undergoing major surgeries, such as joint replacement, which can cause significant stress, are at risk for adrenal crisis. The probability of mortality is high without proper treatment. The results of the present study revealed a 60% prevalence of adrenal insufficiency, a rate much higher than previously reported. Moreover, none of the patients in this study had previously been diagnosed with adrenal insufficiency.
In this study, patients with adrenal insufficiency had significantly higher rates of injection and oral steroid use than the control group. Additionally, a history of steroid injections into the joint or spine was a predictive factor for adrenal insufficiency, whereas a history of oral steroid use was not. Of the 200 included patients, 21 had taken oral steroids, including 6 and 15 who were using hydrocortisone and prednisolone, respectively. The hydrocortisone doses were 10–15 mg/day, while those for prednisolone were ≥10 mg/day, exceeding the physiological dose. Most of these subjects had a history of using steroids for ≥3–4 weeks. Due to the diversity in steroid types, dosages, and durations of use, statistical analysis was not feasible. Previous studies have reported an increased risk of developing adrenal insufficiency in patients taking oral prednisolone at a dose of ≥5 mg/day or oral hydrocortisone at a dose of ≥15 mg for ≥3–4 weeks.20-22) In this study, the prevalence of adrenal insufficiency in patients using steroid inhalers did not differ significantly compared to that in patients not using inhalers. However, both steroid inhalers and ointments carry a risk of inducing adrenal insufficiency.23,24)
Temporary adrenal insufficiency caused by short-term steroid treatment is reversible, with recovery of adrenal function after steroid administration.25) Although this study specifically examined the prevalence of adrenal insufficiency at the time of surgery and did not investigate whether it was permanent or reversible, many patients experienced adrenal insufficiency during the perioperative period. Therefore, paying close attention to postoperative management is crucial in these cases. This study investigated medical conditions that could potentially cause adrenal insufficiency. However, the distribution of these conditions did not differ significantly between the two groups, making it difficult to identify a specific medical condition as the cause of adrenal insufficiency.
The risk of adrenal crisis in patients with adrenal insufficiency is approximately 10 in 100 patient years.26) The primary clinical features of adrenal crisis are hypotension and hypovolemia. However, the initial symptoms such as anorexia, nausea, vomiting, abdominal pain, fatigue, lethargy, fever, and altered consciousness are often nonspecific.6) While the prophylactic administration of steroids in patients with adrenal insufficiency can prevent adrenal crises, the postoperative administration of steroids to patients who have undergone artificial joint surgery may be avoided because of concerns about impaired wound healing and an increased risk of infection. Therefore, a strong conviction that these symptoms are due to an adrenal crisis is necessary to actively initiate steroid treatment. The misdiagnosis of patients with sepsis and subsequent delay in steroid administration can worsen their condition. Most cases of untreated adrenal crises result in death.8,27) Thus, prompt recognition and appropriate treatment of adrenal crises are essential for patient survival. During the study period, five patients with symptoms suggestive of adrenal crisis, including postoperative hypotension, mental changes, and respiratory failure, showed immediate improvement with steroid treatment. The tests for cerebrovascular accidents and pulmonary embolism yielded negative results.
The symptoms of adrenal insufficiency, such as fatigue and loss of appetite, may be nonspecific and may be overlooked in older adult patients with chronic arthropathy. However, the prevalence of these symptoms differed significantly between the two groups in this study. The results of the regression analysis further indicated that a loss of appetite was associated with adrenal insufficiency. Therefore, the surveillance of these symptoms may be helpful in screening for adrenal insufficiency.
This study has several limitations. First, the sample size was small. Considering the known prevalence of adrenal insufficiency, a larger sample size was required in this prospective study. However, a previous pilot study investigating the prevalence of adrenal insufficiency in subjects scheduled for TKA also observed a higher prevalence than previously reported. Secondly, this was a time-zero study. This study only investigated the prevalence of preoperative adrenal insufficiency without determining the effect of adrenal insufficiency on surgical outcomes. We did not provide prophylactic steroid supplementation to patients with adrenal insufficiency. Among patients with preoperative adrenal insufficiency, five exhibited symptoms of adrenal crisis shortly after surgery; however, their condition improved immediately after steroid supplementation. Third, synchronous stimulation testing results may yield false negative results or appear normal in cases of mild disease or with recent onset.28) In mild disease, sufficient adrenal functional cortex may exist to sustain adrenal reserve, enabling a suitable response to the standard dose of synacthen. Similarly, in cases with recent onset, sufficient time may not have elapsed for the adrenal gland to lose its complete function, allowing it to respond to synacthen stimulation. Finally, the number of steroid administrations, an important risk factor for adrenal insufficiency, was investigated based on the patients’ recall. While the oral medications taken by the patients at the time of admission were thoroughly examined, the medications they had previously taken orally and stopped before hospitalization may not have been completely investigated. Due to these limitations, it may be challenging to establish a definitive causal relationship between oral steroid use and the occurrence of adrenal insufficiency within the scope of this study. However, our results revealed adrenal insufficiency in most of the patients who did not receive steroid injections. Therefore, surgeons should be cautious when treating patients with end-stage osteoarthritis.
In conclusion, we observed a high prevalence of adrenal insufficiency in Korean patients hospitalized for TKA due to end-stage osteoarthritis. Recent steroid injections were causally related to the development of adrenal insufficiency. Therefore, adrenal function should be assessed preoperatively to prevent postoperative complications related to adrenal insufficiency.
ACKNOWLEDGMENTS
Table 1.
Demographics of the subjects between the two groups
| Group 1 (n=120) | Group 2 (n=80) | p-value | |
|---|---|---|---|
| Age (y) | 73.4 ± 6.7 | 71.8 ± 7.2 | 0.118a) |
| Sex | 0.077b) | ||
| Male | 23 | 24 | |
| Female | 97 | 56 | |
| Bilaterality of TKA | 0.299b) | ||
| Unilateral | 57 | 44 | |
| Bilateral | 63 | 36 | |
| Biochemistry tests | |||
| Blood urea nitrogen | 16.9±5.3 (7.0–36.1) | 17.4±7.1 (5.5–50.5) | 0.544a) |
| Creatinine | 0.76±0.20 (0.41–1.42) | 0.83±0.51 (0.43–4.58) | 0.219a) |
| Total protein | 6.9±0.4 (6.0–7.8) | 7.0±0.4 (6.1–8.1) | 0.243a) |
| Albumin | 4.2±0.3 (3.3–4.7) | 4.3±0.2 (3.6–4.7) | 0.139a) |
| Aspartate transaminase | 28±12 (16–104) | 29±14 (13–100) | 0.713a) |
| Alanine transaminase | 25±12 (8–68) | 28±25 (8–156) | 0.367a) |
| Sodium (Na) | 143±2 (137–147) | 142±4 (122–147) | 0.146a) |
| Potassium (K) | 4.2±0.4 (3.4–5.7) | 4.2±0.3 (3.4–5.3) | 0.748a) |
| Bone mineral density | -0.7±1.6 | -0.5±1.4 | 0.385a) |
| Body mass index (kg/m2) | 27.08±3.68 | 26.95±3.48 | 0.921a) |
| ASA classification | 0.491b) | ||
| 1 | 4 | 1 | |
| 2 | 97 | 64 | |
| 3 | 19 | 14 | |
| 4 | 0 | 1 | |
| Underlying diseases | 0.512b) | ||
| Asthma | 10 | 8 | |
| Chronic obstructive pulmonary disease | 1 | 4 | |
| Connective tissue disease | 1 | 1 | |
| Skin disease | 1 | 0 | |
| Thyroid disease | 9 | 3 | |
| Hypertension | 97 | 59 | |
| Diabetes mellitus | 39 | 31 | |
| Tuberculosis | 1 | 2 | |
| None | 12 | 10 |
Table 2.
Basal ACTH, basal and stimulated blood cortisol level
| Group 1 (n=120) | Group 2 (n=80) | p-value | |
|---|---|---|---|
| Basal plasma ACTH level (pg/mL) | 16.88±15.51 | 24.58±19.97 | 0.003a) |
| Serum cortisol level (µg/dL) | |||
| Before synacthen stimulation | 4.08±3.18 | 7.54±4.49 | <0.001a) |
| 30 minutes after stimulation | 11.31±3.87 | 17.61±1.95 | <0.001a) |
| 60 minutes after stimulation | 13.39±4.20 | 20.00±2.05 | <0.001a) |
Table 3.
The proportion of subjects with a history of steroid use within 3 months prior to TKA in each group
| Group 1 (n=120) | Group 2 (n=80) | p-value | |
|---|---|---|---|
| Steroid injections | <0.001c) | ||
| Yes (mean cumulative dosage)a) | 93 (17.9±9.2 mg) | 44 (13.4±5.9 mg) | |
| No | 27 | 36 | |
| Oral steroid administration | 0.038c) | ||
| Yes (mean dosage per day)b) | 17 (7.5 mg, 2.5–15.0) | 4 (11.3 mg, 2.5–15.0) | |
| No | 103 | 76 | |
| Steroid inhaler | 0.466c) | ||
| Yes | 6 | 6 | |
| No | 114 | 74 |
a) To standardize the potency of various steroid injections, they were converted to an equivalent dose of betamethasone with the same potency for the analysis.
Table 4.
Systemic symptoms of steroid depletion between the two groups
| Group 1 (n=120) | Group 2 (n=80) | p-value | |
|---|---|---|---|
| Fatigue | 78 | 37 | <0.001a) |
| Loss of appetite | 53 | 15 | |
| None | 26 | 38 |
Table 5.
Logistic regression analysis for variables with significant differences between the two groups
| β | SE β | Wald’s χ2 | df | p-value | Exp(β)a) | |
|---|---|---|---|---|---|---|
| Total injection within 3 months prior to surgery | 0.345 | 0.112 | 9.458 | 1 | 0.002 | 1.412 |
| Daily oral steroid within 3 months prior to surgery | 0.002 | 0.267 | 0.000 | 1 | 0.993 | 1.002 |
| Fatigue | 0.405 | 0.323 | 1.573 | 1 | 0.210 | 1.499 |
| Loss of appetite | 0.938 | 0.361 | 6.776 | 1 | 0.009 | 2.556 |
REFERENCES
2. Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab 2010;95:5110–21.



3. Johnston PC, Lansang MC, Chatterjee S, Kennedy L. Intra-articular glucocorticoid injections and their effect on hypothalamic-pituitary-adrenal (HPA)-axis function. Endocrine 2015;48:410–6.



4. Reid DM, Eastmond C, Rennie JA. Hypothalamic-pituitary-adrenal axis suppression after repeated intra-articular steroid injections. Ann Rheum Dis 1986;45:87.



5. Yamamoto T. Latent adrenal insufficiency: concept, clues to detection, and diagnosis. Endocr Pract 2018;24:746–55.


6. Dineen R, Thompson CJ, Sherlock M. Adrenal crisis: prevention and management in adult patients. Ther Adv Endocrinol Metab 2019;10:2042018819848218.




7. Rathbun KM, Nguyen M, Singhal M. Addisonian crisis. Treasure Island, FL: StatPearls; 2022.
8. Meyer G, Badenhoop K. Addisonian crisis: risk assessment and appropriate treatment. Dtsch Med Wochenschr 2018;143:392–6.


9. Rushworth RL, Torpy DJ. A descriptive study of adrenal crises in adults with adrenal insufficiency: increased risk with age and in those with bacterial infections. BMC Endocr Disord 2014;14:79.




10. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crises in older patients. Lancet Diabetes Endocrinol 2020;8:628–39.


11. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502.



12. Davis MP, Khoshknabi D, Walsh D, Lagman R, Karafa MT, Aktas A, et al. Four-item fatigue screen: replacing the Brief Fatigue Index. Am J Hosp Palliat Care 2013;30:652–6.



13. Hui D, Bruera E. The Edmonton symptom assessment system 25 years later: past, present, and future developments. J Pain Symptom Manage 2017;53:630–43.


14. Chang YJ, Lee JS, Lee CG, Lee WS, Lee KS, Bang SM, et al. Assessment of clinical relevant fatigue level in cancer. Support Care Cancer 2007;15:891–6.



15. Blauwhoff-Buskermolen S, Ruijgrok C, Ostelo RW, de Vet HC, Verheul HM, de van der Schueren MA, et al. The assessment of anorexia in patients with cancer: cut-off values for the FAACT-A/CS and the VAS for appetite. Support Care Cancer 2016;24:661–6.



16. Molfino A, Kaysen GA, Chertow GM, Doyle J, Delgado C, Dwyer T, et al. Validating appetite assessment tools among patients receiving hemodialysis. J Ren Nutr 2016;26:103–10.


17. Hamilton DD, Cotton BA. Cosyntropin as a diagnostic agent in the screening of patients for adrenocortical insufficiency. Clin Pharmacol 2010;2:77–82.


18. Doyle DJ, Goyal A, Garmon EH. American Society of Anesthesiologists Classification. Treasure Island, FL: StatPearls; 2022.
19. Noh JH, Jung HW, Ga H, Lim JY. Ethical guidelines for publishing in the Annals of Geriatric Medicine and Research. Ann Geriatr Med Res 2022;26:1–3.




20. Sagar R, Mackie S, W Morgan A, Stewart P, Abbas A. Evaluating tertiary adrenal insufficiency in rheumatology patients on long-term systemic glucocorticoid treatment. Clin Endocrinol (Oxf) 2021;94:361–70.



21. Erskine D, Simpson H. Exogenous steroids, adrenal insufficiency and adrenal crisis: who is at risk and how should they be managed safely [Internet]. Bristol, UK: Society for Endocrinology; 2021 [cited 2023 Nov 30]. Available from: https://mg.salisbury.nhs.uk/media/2864/spssfe_supporting_sec_-final_hls-19022021-2-1.pdf.
22. Alexandraki KI, Kaltsas GA, Chrousos GP. Adrenal suppression. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth, MA: MDText.com; 2000 [cited 2023 Nov 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279047/.
23. Broersen LH, Pereira AM, Jørgensen JO, Dekkers OM. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab 2015;100:2171–80.



25. Laugesen K, Broersen LH, Hansen SB, Dekkers OM, Sorensen HT, Jorgensen JO. Management of endocrine disease: glucocorticoid-induced adrenal insufficiency. Replace while we wait for evidence? Eur J Endocrinol 2021;184:R111–22.


26. Puar TH, Stikkelbroeck NM, Smans LC, Zelissen PM, Hermus AR. Adrenal crisis: still a deadly event in the 21st century. Am J Med 2016;129:339.

27. Claessen KM, Andela CD, Biermasz NR, Pereira AM. Clinical unmet needs in the treatment of adrenal crisis: importance of the patient's perspective. Front Endocrinol (Lausanne) 2021;12:701365.



28. Khare S, Anjum F. Adrenocorticotropic hormone test. Treasure Island, FL: StatPearls; 2020.
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 973 View
- 86 Download
-
Related articles in
Ann Geriatr Med Res -
Dexmedetomidine Sedation in an Old Patient for Revision Total Hip Arthroplasty2012 March;16(1)
The Prevalence of Sarcopenia in Korean Hospitalized Elderly2015 December;19(4)