|
 |
- Search
| Ann Geriatr Med Res > Volume 27(2); 2023 > Article |
|
Abstract
Background
No comprehensive assessment of the quality of medications used in older outpatients has been conducted in Thailand. This study aimed to ascertain the prevalence of and factors contributing to older outpatients' use of potentially inappropriate medications (PIMs).
Methods
This cross-sectional study retrospectively assessed the prescriptions of older (âĽ60 years) outpatients at a secondary-care hospital. For PIM identification, the 2019 American Geriatric Society (AGS) Beers criteria were applied, considering all five PIM categories: PIM category I (medications that are potentially inappropriate in most older adults), II (disease-/syndrome-exacerbating drugs), III (medications that should be used with caution), IV (clinically important drugâdrug interactions), and V (medications that should be avoided or have their dosage reduced based on renal function).
Results
This study included 22,099 patients (mean age, 68.86Âą7.64 years). Nearly three-fourths of patients were prescribed PIMs, with 68.90%, 7.68%, 44.23%, 15.66%, and 3.05%, respectively, receiving category IâV medications. The positive factors associated with PIM use included female sex (odds ratio [OR]=1.08; 95% confidence interval [CI], 1.01â1.16), age âĽ75 years (OR=1.10; 95% CI, 1.01â1.21), polypharmacy (OR=10.21; 95% CI, 9.31â11.21), âĽ3 diagnostic categories (OR=2.31; 95% CI, 2.14â2.50), and âĽ3 chronic morbidities (OR=1.46; 95% CI, 1.26â1.68). The negative factor associated with PIM use was a comorbidity score of âĽ1 (OR=0.78; 95% CI, 0.71â0.86).
The number of older adults is markedly increasing worldwide. Thailand is currently an aging society, and older people constitute approximately 19% of the total population.1) Because they are at a higher risk of adverse drug events (ADEs), older adults must be especially cautious when taking medications.2) According to the Health Product Vigilance Center (HPVC), 26.62% of ADE reports were from older adults, accounting for 17.90% of drug-related mortalities.3)
Many medications are considered potentially inappropriate for use by older adults and are frequently referred to as potentially inappropriate medications (PIMs).4) Several sets of PIM detection criteria are currently available for use as assessment or screening tools to reduce the use of PIMs in older patients. 5-8) The American Geriatric Society (AGS) Beers criteria is a well-known and frequently cited tool among existing criteria. The AGS Beers criteria contain drug-specific and explicit criteria that address various aspects of inappropriate prescriptions.8-10) The 2019 AGS Beers criteria, the most recent version, proposes five PIM categories, including PIM categories I (medications that may be inappropriate in most older adults), II (medications that may worsen the disease or syndrome), III (medications that should be used with caution), IV (clinically significant drugâdrug interactions), and V (medications that should be avoided or whose dosage should be adjusted based on renal function). For each criterion, the risk rationale, recommendation, and strength of recommendation are clearly stated. For example, first-generation antihistamines are strongly advised to be avoided because of their strong anticholinergic effects.11) The AGS Beers criteria are applicable to all levels of healthcare services, both inpatient and outpatient, and are unaffected by the patient's level of frailty.10,12)
Secondary-care hospitals may have limited healthcare resources, such as geriatricians and clinical pharmacists, limited alternative medications, and less geriatric clinics, resulting in insufficient medication reviews. There are no reports on the quality of medication use in older outpatients receiving both short- and long-term care from secondary-care hospitals in Thailand. The current study sought to ascertain the prevalence of PIM use as determined by all aspects of the 2019 AGS Beers criteria as well as the factors associated with PIM use.
This study used a descriptive cross-sectional design. Data on older outpatients were obtained from the electronic medical record (EMR) database of a public hospital. The study hospital was a 231-bed secondary-care hospital located in the metropolitan district of Phayao Province, Thailand, that serves patients from the hospital district and five surrounding districts. Data from the EMR database were retrieved by the hospital staff working at the hospital data center.
We selected this hospital as the study setting because it offers a comprehensive EMR database. All data, including demographics, diagnoses, laboratory results, and prescriptions, were recorded in the EMR database. All medications prescribed in the outpatient and inpatient departments were recorded separately in the EMR database.
The study protocol was reviewed and approved by the Human Ethics Committee of the University of Phayao (Code No. UP-HEC 1.1/013/65, Date: June 8, 2022) and the Institutional Review Board of the study hospital (Code No. 006/2565, Date: July 8, 2022) before data collection.
This study included all patients aged âĽ60 years (referred to as âolderâ Thais)13) who visited the outpatient department (OPD) between January 1, 2020, and December 31, 2021 (a 2-year period). Patients lacking data on their diagnoses or prescriptions or those who were only administered the coronavirus disease 2019 (COVID-19) vaccine were excluded from the study.
Also, this study complied the ethical guidelines for authorship and publishing in the Annals of Geriatric Medicine and Research.14)
The EMR database contains all patient and medical data, including demographics (i.e., sex, age, age groups of 60â74 and âĽ75 years), health insurance (universal coverage [UC] and non-UC), and clinical data (i.e., diagnostic categories, chronic morbidity, and the comorbidity score calculated for each patient using the Charlson Comorbidity Index]).15-17) The International Classification of Diseases, 10th revision (ICD-10) coding schemes proposed by Glasheen et al.18) and Tonelli et al.19) were used to identify all chronic morbidities; OPD prescriptions (i.e., the number of prescriptions received by the patient between the index date, defined as the first date of an OPD visit with a prescription, and December 31, 2021); and polypharmacy, defined as a prescription containing âĽ5 medications. Patients were assigned to the polypharmacy group if they had at least one prescription with polypharmacy.
We primarily applied the 2019 AGS Beers criteria for the identification of all five PIM categories. All medications or medication classes were extracted from Tables 2â7 of the 2019 AGS Beers criteria and checked for availability in the study hospital. Among these, 102 PIM-related medications were identified. Hospital drug codes relevant to each medication were used to extract medications from electronic OPD prescriptions.
The PIM assessment considered the conditions related to PIM. Insulin regimens containing only short- or rapid-acting insulin (e.g., regular insulin) and no concurrent use of basal or long-acting insulin (e.g., insulin mix 70/30 or neutral protamine Hagedorn [NPH] insulin) were classified as category I PIMs. Only medications prescribed to patients with any of the 10 targeted diseases or syndromes, such as opioid use in patients with a history of falls or fractures, were identified as PIM category II. Low-dose aspirin use was considered PIM category III based on the age group (60â69 vs. âĽ70 years). Only the co-prescription of objects and interacting drugs, such as opioids and gabapentin, was identified as PIM category IV. Only medication prescribed to patients with renal dysfunction was identified as PIM category V, such as colchicine use in a patient with an estimated glomerular filtration rate (eGFR) of <30 mL/min/1.73 m2 on the date of the medication prescription. Additionally, a group of medications with high anticholinergic effects (Table 7 of the Beers criteria), listed as PIM categories I and II (delirium, dementia/cognitive impairment, and lower urinary tract symptoms [LUTS] or benign prostatic hyperplasia [BPH]) and IV (concurrent use of two anticholinergics), were assessed.
We expressed continuous variables with normal distributions as meanÂąstandard deviation and used independent-sample t-tests to compare PIM use with no PIM use. We expressed continuous variables with non-normal distributions as median and interquartile range (Q1âQ3), and used MannâWhitney U tests to compare PIM use with no PIM use. We expressed categorical variables as frequencies and percentages and used chi-square or Fisher exact tests for comparisons between PIM use and no PIM, as applicable.
This study considered the patient as the unit of analysis. We calculated the prevalence of overall PIM use, PIM category use, and individual PIM use as a percentage by dividing the number of patients prescribed PIM by the total number of patients. To reflect the consistency of PIM use, we calculated the number of times a PIM was prescribed, expressed as a median and interquartile range (Q1âQ3) for each PIM.
To determine the factors associated with PIM use, we performed binary logistic regression analysis to calculate crude odds ratio (OR), adjusted odds ratio (aOR), and 95% confidence interval (CI). Factors with p<0.2 in the univariate analysis were included in the adjustment analysis. The variance inflation factor (VIF) for each factor was calculated to diagnose multicollinearity (high correlation of two independent variables). Factors with a VIF >5 were excluded from the model.20) We then identified the associated factors using a backward elimination method, in which the factor with the least significance was discarded at each step until all remaining factors in the model had p<0.05.
We used STATA 14.0 (StataCorp LLC, College Station, TX, USA) to perform all the statistical analyses. All the hypothesis tests were two-tailed. Statistical significance was set at p<0.05.
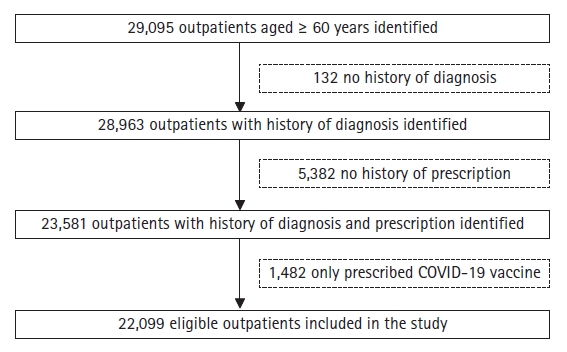
Fig. 1 illustrates the patient recruitment process. Initially, 29,095 outpatients aged 60 years and older were enrolled. Due to ineligibility, 6,996 patients were excluded (132, 5,382, and 1,482 patients lacked data on diagnosis and prescription and were prescribed only the COVID-19 vaccine, respectively). Thus, this study included 22,099 older outpatients.
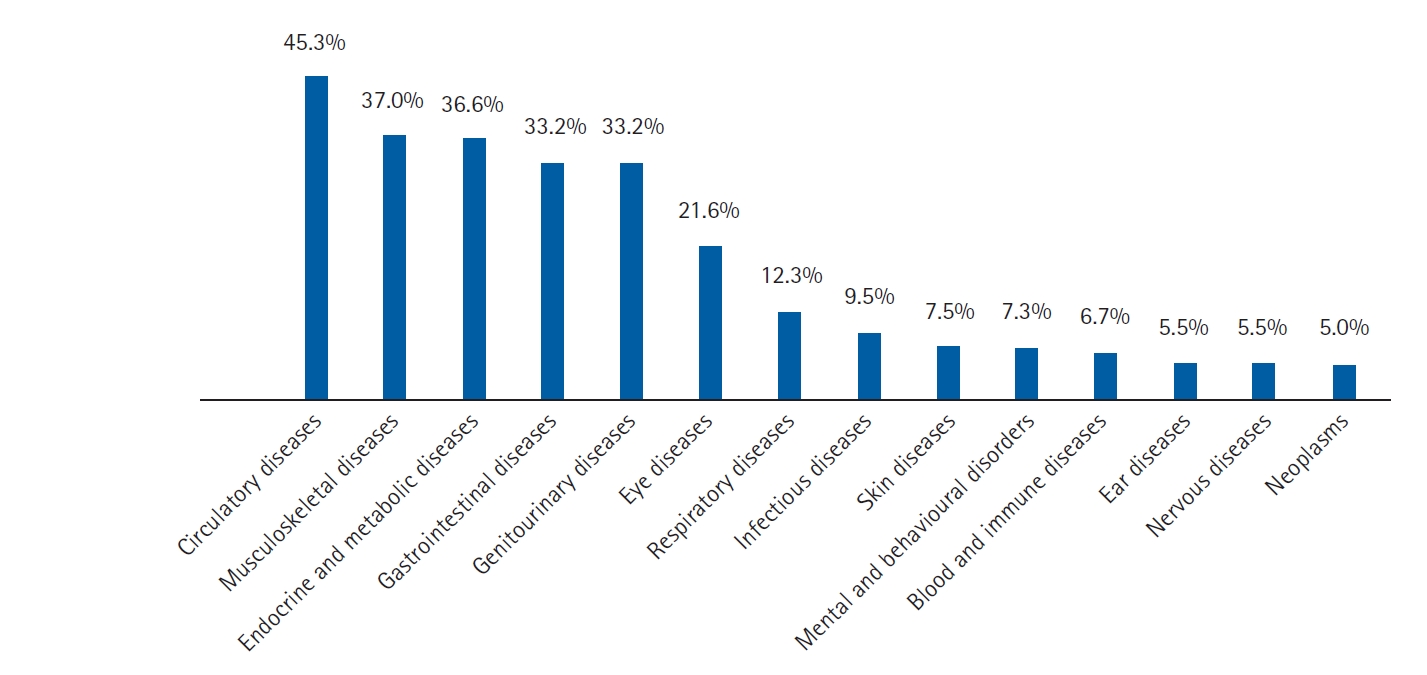
Table 1 presents the characteristics of the study participants. Most of the patients (52.92%) were female, and the average age was 68.86Âą7.64 years. The primary source of health insurance was UC (70.81%). Most patients (71.53%) had âĽ3 diagnostic categories, with a median of 4 (IQR 2â6). As illustrated in Fig. 2, cardiovascular disorders were the most common (45.3%), followed by musculoskeletal diseases (37.0%) and endocrine and metabolic diseases (36.6%). Approximately one-fifth of patients had ⼠3 chronic morbidities, with a median of 1 (IQR 0â2). The most common condition was hypertension (38.11%), followed by mild-to-moderate chronic renal disease (21.58%) and diabetes without complications (14.84%). PIM users had a significantly higher prevalence of polypharmacy, chronic morbidities, and comorbidity scores than non-users. More than half (53.86%) the patients received at least one polypharmacy prescription.
Table 2 summarizes the general prevalence of PIM use in each PIM category. Nearly three-fourths of patients (74.70%) were prescribed at least one PIM, with 29.80%, 25.19%, 15.75%, 3.56%, and 0.39% prescribed 1, 2, 3 , 4 and 5 categories respectively. The distribution of PIM in each category is detailed in Supplemental Tables S1âS5.
For category I PIMs (medications that may be inappropriate for most older adults), except for nifedipine immediate release, amobarbital, ethinyl estradiol, and dipyridamole, all PIMs were prescribed to patients. Of these patients, 68.90% were administered at least one PIM. The top five most prescribed PIMs were orphenadrine (38.80%), omeprazole (29.16%), naproxen (11.82%), dimenhydrinate (11.43%), and lorazepam (10.26%). More than half the patients (53.39%) were prescribed at least one anticholinergic medication (Supplement Table S1).
For category II PIMs (medications that may worsen the disease or syndrome), except for urinary incontinence (all forms) in women, PIMs were prescribed for all ten targeted diseases or syndromes. A total of 7.68% of the patients were administered at least one PIM. The most frequently prescribed PIMs were observed in patients with a history of falls or fractures, with tramadol being the most prescribed (3.76%), followed by lorazepam (1.16%), amitriptyline (0.93%), and morphine (0.57%). In cases of other diseases or syndromes, patients with dementia, cognitive impairment, delirium, LUTS, or BPH received anticholinergics. Patients with heart failure and chronic kidney disease stage 4 or higher were prescribed all types of non-steroidal anti-inflammatory drugs (NSAIDs), including non-specific cyclooxygenase (COX), COX-2-selective and COX-2-specific inhibitors, whereas patients with a history of gastric or duodenal ulcers were prescribed non-specific COX and COX-2-selective inhibitors (Supplemental Table S2).
Category III PIMs (medications that should be used with caution) were prescribed to 44.23% of patients. Tramadol was the most commonly prescribed (18.74%), followed by low-dose aspirin (14.73%) and dextromethorphan (8.06%). Nearly half (46.16%) the patients receiving low-dose aspirin were aged >70 years (Supplemental Table S3).
Among category IV PIMs (clinically significant drugâdrug interactions), a pair of objects and an interacting drug was prescribed to 15.66% of patients. The concurrent use of two anticholinergics was most common (9.33%), most commonly amitriptyline and orphenadrine (3.54%), followed by dimenhydrinate and orphenadrine (2.24%). The four most prescribed PIM were orphenadrine-based regimens. Among opioid users, the concurrent use of tramadol and gabapentin was the most common (1.78%), followed by tramadol and lorazepam (1.01%). Although combinations of the three central nervous system (CNS)-active drugs were slightly more common, each regimen was consistently prescribed. Warfarin and other drugs have a minor interaction. Nevertheless, the concomitant use of warfarin and low-dose aspirin was administered to 64 individuals, with a median of 5 prescriptions (IQR 2â9) (Supplementary Table S4).
Only 3.05% of patients were prescribed category V PIMs (medications that should be avoided or whose dosage should be adjusted based on renal function). Colchicine (1.57%), tramadol (0.82%), and spironolactone (0.20%) were the most commonly administered drugs to patients with significantly compromised renal function (eGFR <30 mL/min/1.73 m2) (Supplementary Table S5).
Table 3 presents the results of univariate and multivariate analyses of the factors associated with PIM use. Women exhibited an OR of 1.08 (95% CI, 1.01â1.16) compared to that in men. Patients aged âĽ75 years exhibited an OR of 1.10 (95% CI, 1.01â1.21) compared to those aged 60â74 years. Polypharmacy exhibited an OR of 10.21 (95% CI, 9.31â11.21) compared to non-polypharmacy. Patients with âĽ3 diagnostic categories exhibited an OR of 2.31 (95% CI, 2.14â2.50) compared to those with 1â2 diagnostic categories. Patients with âĽ3 chronic morbidities exhibited an OR of 1.46 (95% CI, 1.26â1.68) compared to those with 0â2 chronic morbidities. Patients with a comorbidity score of âĽ1 exhibited an OR of 0.78 (95% CI, 0.71â0.86) compared to those with a score of 0.
This study evaluated the quality of medication use in older outpatients receiving care at a single secondary-care hospital. According to the 2019 AGS Beers criteria, PIM use is particularly prevalent among older outpatients, accounting for up to three-fourths being prescribed PIM. All five PIM categories were identified. Polypharmacy was the most significant predictor of PIM use.
The overall prevalence of PIM use among our outpatients in this secondary-care hospital was up to 74.70%, which was greater than that in hospitalized patients in tertiary-care hospitalsâ61.9% in a study by Sharma et al.21) and 64.80% in a study by He et al.22) Secondary-care hospitals may have fewer healthcare resources such as alternative drugs, geriatricians, clinical pharmacists, and geriatric clinics, compared to tertiary-care hospitals, resulting in insufficient quality of geriatric care. Outpatients are more likely to use PIMs than hospitalized patients because they have more morbidities that require long-term treatment in the OPD. In our study, more than half the patients had âĽ3 diagnostic categories and up to five prescriptions during the study period.
Category I PIMs (medications that may be inappropriate for most older adults) showed the highest prevalence of PIM use (68.90%), consistent with both foreign and Thai study results.16,21-26) In those studies, the most prescribed PIM classes were first-generation antihistamines, benzodiazepines (BZDs), proton-pump inhibitors (PPIs), antidepressants, and oral NSAIDs. In this study, dimenhydrinate, chlorpheniramine, and hydroxyzine were frequently used as first-generation antihistamines in older Thai patients, consistent with the results of previous Thai studies.16,26,27) Lorazepam was the most prescribed BZD (10.26%), consistent with previous Thai research.16,26) Although short-acting BZDs appear to be safer than long-acting BZDs (older adults have slower metabolism of long-acting agents), the Beers criteria strongly advise avoiding all BZDs because they can increase the risk of cognitive impairment, delirium, falls, fractures, and motor vehicle accidents in older adults.11) As previously reported, omeprazole was frequently prescribed as a PPI in hospitals.21,22,24) The Beers criteria strongly advise against using PPIs for >8 weeks unless in high-risk patients (e.g., chronic use of oral glucocorticoids or NSAIDs) or for approved indications (e.g., erosive esophagitis) because their use can increase the risk of Clostridium difficile infection, bone loss, and fractures.11) PPIs may be prescribed inappropriately, with unapproved indications, co-prescriptions with glucocorticosteroids, and excessive dosages.28,29) Furthermore, older age is a predictor of inappropriately initiated PPI use, with an OR of 1.03 (95% CI, 1.03â1.03).30) In our study, amitriptyline was the most commonly prescribed antidepressant, consistent with the findings of previous Thai studies.16,26,27) The Beers criteria strongly advise against antidepressant use due to the increased risk of highly anticholinergic effects, sedation, and orthostatic hypotension.11) Most studies on PIM safety reported associations between both non-COX and COX-2-selective NSAID use and increased risks of gastrointestinal bleeding, high blood pressure, and kidney injury.16,22,26,27,31) Thus, the Beers criteria strongly advise against the long-term (âĽ3 months) use of NSAIDs unless other alternatives are ineffective, and the use of a gastroprotective agent to reduce the risk.11) Interestingly, our results revealed that orphenadrine, a skeletal muscle relaxant, was the most commonly prescribed PIM. Most muscle relaxants are poorly tolerated by older adults due to their strong anticholinergic effects, sedation, and increased risk of fracture.11)
Category II PIMs (medications that may worsen the disease or syndrome) were found in all 10 diseases or syndromes except for urinary incontinence (all types) in women. Patients with a history of falls or fractures had the highest prevalence of use (4.77%), with tramadol being the most commonly prescribed medication (3.76%), consistent with the report by Walker et al.32) The 2019 AGS Beers criteria recommend avoiding opioid use in older adults with a history of falls or fractures as these medications can cause ataxia, impaired psychomotor function, syncope, and additional falls.11) The patients in the present study were prescribed lorazepam, risperidone, haloperidol, and anticholinergics (orphenadrine and amitriptyline) for the treatment of delirium, whereas Sharma et al.21) reported the use of ranitidine and hydrocortisone. Lorazepam, risperidone, and anticholinergics (orphenadrine, trihexyphenidyl, and dimenhydrinate) were prescribed to patients with dementia or cognitive impairment, consistent with previous reported findings.21,25,33) The 2019 Beers criteria recommend avoiding the prescription of anticholinergics, antipsychotics, and BZDs in older patients with delirium or dementia as these medications can worsen the disease.11) Pioglitazone and all types of NSAIDs were prescribed to our patients with heart failure, consistent with the results of a study by Duangsong et al.,34) who reported a 19.67% prevalence of PIMs in patients with heart failure based on the 2019 AGS Beers criteria.
Antipsychotics (haloperidol and risperidone), diuretics (furosemide, spironolactone, and hydrochlorothiazide), selective serotonin reuptake inhibitors (SSRIs) (sertraline and fluoxetine), tricyclic antidepressants (TCAs) (amitriptyline), and tramadol were frequently prescribed among category III PIMs (medications that should be used with caution). These medication classes should be used with caution because they may aggravate or cause inappropriate antidiuretic hormone secretion (SIADH) or hyponatremia.11) He et al.22) reported that tramadol was the most commonly used of these medications. Diuretics and SSRIs were the other two PIM classes exhibiting frequent use, consistent with previous research findings.22,24,25) Because heart failure is commonly diagnosed in older patients, diuretics are a commonly prescribed PIM.35) Because low-dose aspirin provides no net benefit to older adults with cardiovascular risk factors when used for primary prevention, the Beers criteria strongly advise caution in using low-dose aspirin in adults aged >70 years to avoid the risk of major bleeding.11) Although we could not directly assess the appropriateness of low-dose aspirin use, up to 46.16% of aspirin users were >70 years of age.
We observed almost all drugâdrug interactions listed in PIM category IV (clinically significant), with anticholinergics being the most concurrent prescriptions. Because of the increased risk of cognitive decline, the Beers criteria strongly recommend limiting the number of anticholinergic drugs used concurrently.11) The Beers criteria also strongly advise against combining NSAIDs and glucocorticoids because they can increase the risk of peptic ulcer disease or gastrointestinal bleeding.11) We observed the interaction between NSAIDs and glucocorticoids (e.g., naproxen + prednisolone), consistent with a study by Sharma et al.21) that reported the interaction of hydrocortisone and ketorolac. Except for the use of warfarin and low-dose aspirin, we observed no concomitant use of warfarin and NSAIDs. A Thai study reported the prescription of this combination therapy to 9.2% of patients with non-valvular atrial fibrillation.36) However, a recent study found that adding low-dose aspirin to patients receiving warfarin without a clear indication for aspirin (e.g., mechanical heart valve replacement, recent percutaneous coronary intervention, or acute coronary syndrome) may increase the risk of bleeding without additional therapeutic benefits.37) Thus, close monitoring for bleeding is required when these medications are used together.11)
We observed the use of many category V PIMs (medications that should be avoided or whose dosage should be adjusted based on renal function), including gabapentin, enoxaparin, ranitidine, tramadol, dabigatran, and spironolactone, consistent with previous reports.21,23,38) Colchicine was also mentioned in our research. Because it has been linked to gastrointestinal, neuromuscular, and bone marrow toxicity, the Beers criteria strongly recommend lowering the dose.11)
Female sex is frequently identified as a patient-related factor.23,25) In this study, individuals aged âĽ75 years were more likely to be prescribed PIMs, consistent with the results of earlier studies, as various morbidities, including sleep problems, delirium, dementia, chronic pain, and heart failure, are typically diagnosed in this age group.25,26) Polypharmacy was the most important component identified in previous studies on PIM.22,24,25,33) The likelihood of PIM use can increase with polypharmacy. Individuals with âĽ3 diagnostic categories or chronic morbidities in the present study were more likely to be prescribed PIMs, consistent with previous research findings.25,26) Individuals with comorbidity scores of âĽ1 were less likely to be administered PIMs and, according to Tian et al.,25) physicians should use caution while providing PIMs to patients with higher illness burdens.
Our study has several advantages. First, we reported the prevalence of PIM use detected using the 2019 AGS Beers criteria, which considers all five PIM categories. Second, we used real-world data from a large population of older adults to measure PIM use. Third, we evaluated numerous prescriptions per patient (rather than only one), which increased the likelihood of identifying PIM use. Finally, we reported the use of PIMs in terms of frequency and continuity to provide a better understanding.
Our study had some limitations. First, because only one hospital was analyzed, it could not cover all PIM-related medications; therefore, this study did not evaluate medications that might be available in other hospitals. Second, we were unable to evaluate the connection between individual chronic morbidity and PIM use because PIMs can be used for various indications. Instead, we determined the specific groups associated with PIM use. Third, because we evaluated the prescriptions received by each patient from the index date to the end of 2021, some patients had a short study period for PIM assessment. Moreover, the patients may have received PIM from sources other than hospitals. Thus, PIM use may have been more prevalent in both cases than indicated in this study. Fourth, because we analyzed numerous PIM categories, we were unable to exclude PIM-related drugs from the patientsâ prescriptions. When polypharmacy was identified, PIM-related drugs were included in the drug count. Our results showed that polypharmacy was the most important factor influencing PIM use, with the highest OR. Finally, our findings should not be applied to other contexts involving various medications and prescription practices.
In conclusion, both the overall PIM use and the use of each PIM category remained high in older Thai outpatients. Compliance with the Beers criteria is necessary to reduce all aspects of inappropriate prescriptions. These measures should be aimed at individuals at high risk of using PIMs, such as those ⼠75 years of age, with several morbidities, and on multiple medications.
ACKNOWLEDGMENTS
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4235/agmr.23.0036.
Table 1.
Characteristics of the study participants
Table 2.
PIM use distribution by PIM category (n=22,099)
| PIM categorya) | Prevalence (%) | n (%) | Number of times PIM prescribed |
|---|---|---|---|
| I. Medications that may be inappropriate in most older adults | 68.90 (n=15,226) | ||
| âOrphenadrine | 8,574 (38.80) | 2 (1â3) | |
| âOmeprazole | 6,445 (29.16) | 2 (1â6) | |
| âNaproxen | 2,612 (11.82) | 1 (1â2) | |
| âDimenhydrinate | 2,526 (11.43) | 2 (1â2) | |
| âLorazepam | 2,268 (10.26) | 3 (1â6) | |
| II. Medications that may worsen the disease or syndrome | 7.68 (n=1,697) | ||
| âTramadol (falls or fracture) | 831 (3.76) | 1 (1â2) | |
| âOrphenadrine (LUTS, BPH) | 396 (1.79) | 2 (1â4) | |
| âLorazepam (falls or fracture) | 256 (1.16) | 9 (1â9) | |
| âAmitriptyline (falls or fracture) | 205 (0.93) | 1 (1â2) | |
| âMorphine (falls or fracture) | 125 (0.57) | 1 (1â1) | |
| III. Medications that should be used with caution | 44.23 (n=9,775) | ||
| âTramadol | 4,141 (18.74) | 1 (1â2) | |
| âDextromethorphan | 1,781 (8.06) | 1 (1â2) | |
| âAmitriptyline | 1,674 (7.58) | 1 (1â3) | |
| âFurosemide | 1,655 (7.49) | 6 (2â9) | |
| âLow dose aspirin (age âĽ70) | 1,503 (6.80) | 6 (3â7) | |
| IV. Drug-drug interactions that are clinically significant | 15.66 (n=3,460) | ||
| âAmitriptyline + orphenadrine | 783 (3.54) | 1 (1â2) | |
| âDimenhydrinate + orphenadrine | 496 (2.24) | 1 (1â1) | |
| âTramadol + gabapentin | 393 (1.78) | 1 (1â2) | |
| âDoxazosin + furosemide | 299 (1.35) | 4 (2â7) | |
| âHydroxyzine + orphenadrine | 283 (1.28) | 1 (1â2) | |
| V. Medications that should be avoided or whose dosage should be adjusted based on renal function | 3.05 (n=675) | ||
| âColchicine (CrCl <30 mL/min) | 347 (1.57) | 2 (1â5) | |
| âTramadol (CrCl <30 mL/min) | 182 (0.82) | 1 (1â1) | |
| âGabapentin (CrCl <60 mL/min) | 149 (0.67) | 1 (1â2) | |
| âSpironolactone (CrCl <30 mL/min) | 44 (0.20) | 1 (1â2) | |
| âCiprofloxacin (CrCl <30 mL/min) | 32 (0.14) | 1(1â1) | |
| Overall | 74.70 (n=16,507) |
Table 3.
Factors associated with PIM use (n=22,099)
| Factors | Crude OR (95% CI) | p-valuea) | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Female | 1.15 (1.09â1.23) | <0.001 | 1.08 (1.01â1.16) | 0.028 |
| Age, âĽ75 y | 1.42 (1.31â1.53) | <0.001 | 1.10 (1.01â1.21) | 0.038 |
| Universal coverage | 1.04 (0.98â1.11) | 0.213 | - | |
| Polypharmacy | 13.35 (12.28â14.51) | <0.001 | 10.21 (9.31â11.21) | <0.001 |
| Diagnostic categories, âĽ3 | 4.37 (4.10â4.66) | <0.001 | 2.31 (2.14â2.50) | <0.001 |
| Chronic morbidities, âĽ3 | 5.64 (5.03â6.32) | <0.001 | 1.46 (1.26â1.68) | <0.001 |
| Comorbidity score, âĽ1 | 2.98 (2.79â3.19) | <0.001 | 0.78 (0.71â0.86) | <0.001 |
REFERENCES
1. Teerawichitchainan B. Older persons in Thailand: an update from a recent national survey. Asian Popul Stud 2020;16:243â7.

2. Locherty M, Alder S, Caslake R, Mangoni AA. Practical advice for prescribing in old age. Medicine 2021;49:17â21.

3. Health Product Vigilance Center. Spontaneous reports of adverse drug reactions 2020. Bangkok, Thailand: Aksorn Graphic and Design Publishing; 2022.
4. OâConnor MN, Gallagher P, OâMahony D. Inappropriate prescribing: criteria, detection and prevention. Drugs Aging 2012;29:437â52.

5. Kashyap M, Iqbal MZ. A review of screening tools used for the assessment of appropriateness of prescriptionâs among elderly patients. J Pharm Biosci 2014;3:72â9.
6. Kaufmann CP, Tremp R, Hersberger KE, Lampert ML. Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol 2014;70:1â11.



7. Lucchetti G, Lucchetti AL. Inappropriate prescribing in older persons: a systematic review of medications available in different criteria. Arch Gerontol Geriatr 2017;68:55â61.


8. Curtin D, Gallagher PF, O'Mahony D. Explicit criteria as clinical tools to minimize inappropriate medication use and its consequences. Ther Adv Drug Saf 2019;10:2042098619829431.




9. Azermai M, Vander Stichele RR, Elseviers MM. Quality of pharmacotherapy in old age: focus on lists of Potentially Inappropriate Medications (PIMs) : consensus statements from the European Science Foundation exploratory workshop. Eur J Clin Pharmacol 2016;72:897â904.



10. Inocian EP, Felicilda Reynaldo RF, Dillon D, Ignacio EH. Using beers criteria to avoid inappropriate prescribing for older adults. Medsurg Nurs 2021;30:113â7.
11. By the 2019 American Geriatrics Society Beers Criteria(R) Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019;67:674â94.



12. Nicoteri JA. Practical use of the American Geriatric Society Beers Criteria 2019 update. J Nurs Pract 2021;17:789â94.
13. Knodel J, Chayovan N. Older persons in Thailand: a demographic, social and economic profile. Ageing Int 2008;33:3â14.


14. Noh JH, Jung HW, Ga H, Lim JY. Ethical guidelines for publishing in the Annals of Geriatric Medicine and Research. Ann Geriatr Med Res 2022;26:1â3.




15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373â83.


16. Prasert V, Akazawa M, Shono A, Chanjaruporn F, Ploylearmsang C, Muangyim K, et al. Applying the Lists of Risk Drugs for Thai Elderly (LRDTE) as a mechanism to account for patient age and medicine severity in assessing potentially inappropriate medication use. Res Social Adm Pharm 2018;14:451â8.


17. World Health Organization. International Statistical Classification of Diseases and related health problems 10th revision version [Internet]. Geneva, Switzerland: World Health Organization; 2016 [cited 2023 Jun 5]. Available from: https://icd.who.int/browse10/2016/en.
18. Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson comorbidity index: ICD-9 update and ICD-10 translation. Am Health Drug Benefits 2019;12:188â97.


19. Tonelli M, Wiebe N, Fortin M, Guthrie B, Hemmelgarn BR, James MT, et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak 2015;15:31.




20. Senaviratna NA, Cooray TM. Diagnosing multicollinearity of logistic regression model. Asian J Probab Stat 2019;5:1â9.


21. Sharma R, Bansal P, Garg R, Ranjan R, Kumar R, Arora M. Prevalence of potentially inappropriate medication and its correlates in elderly hospitalized patients: a cross-sectional study based on Beers criteria. J Family Community Med 2020;27:200â7.



22. He D, Zhu H, Zhou H, Dong N, Zhang H. Potentially inappropriate medications in Chinese older adults: a comparison of two updated Beers criteria. Int J Clin Pharm 2021;43:229â35.



23. Abdelwahed AA, El-Dahiyat F, Aljawamis D, Al Ajimi J, Bin Rafeea KJ. Potentially inappropriate medications in older adults according to Beers criteria 2019: prevalence and risk factors. Int J Clin Pract 2021;75:e14715.



24. Chinthalapudi SS, Cheeti S, Bajpai A, Deepika S, Thunga G, Rashid M, et al. Prevalence and predictors of potentially inappropriate medication use among elderly patients using updated Beers criteria 2019: a single centered retrospective analysis. Curr Drug Saf 2022;17:24â33.



25. Tian F, Li H, Chen Z, Xu T. Potentially inappropriate medications in Chinese older outpatients in tertiary hospitals according to Beers criteria: a cross-sectional study. Int J Clin Pract 2021;75:e14348.



26. Vatcharavongvan P, Prasert V, Ploylearmsang C, Puttawanchai V. Prevalence and factors that influence potentially inappropriate medication use among Thai elderly in primary care settings. Can Geriatr J 2021;24:332â40.




27. Jenghua K, Sangtong H, Jaiya N, Jaroenteerawit W, Khiewpradang A. The use of potentially inappropriate medications (PIMs) from Thailand criteria among urban community-dwelling elderly: prevalence, PIMs, and factors associated. Thai Bull Pharm Sci 2019;14:49â63.
28. Celik F, Aypak C, Ozdemir A, Gorpelioglu S. Inappropriate prescribing of proton pump inhibitors in outpatient clinics. Gastroenterol Nurs 2021;44:84â91.


29. Liu Y, Zhu X, Li R, Zhang J, Zhang F. Proton pump inhibitor utilisation and potentially inappropriate prescribing analysis: insights from a single-centred retrospective study. BMJ Open 2020;10:e040473.



30. Koggel LM, Lantinga MA, Buchner FL, Drenth JP, Frankema JS, Heeregrave EJ, et al. Predictors for inappropriate proton pump inhibitor use: observational study in primary care. Br J Gen Pract 2022;72:e899â906.



31. Eteraf Oskouei T, Vatankhah E, Najafi M. The status of potentially inappropriate medication prescription by general physicians for the elderly in Tabriz (Iran) according to Beers criteria. Iran J Ageing 2021;16:274â87.

32. Walker BS, Collier BR, Bower KL, Lollar DI, Faulks ER, Matos M, et al. The prevalence of Beers criteria medication use and associations with falls in geriatric patients at a level 1 trauma center. Am Surg 2019;85:877â82.



33. Harasani K, Xhafaj D, Begolli A, Olvera-Porcel MC. Prevalence of potentially inappropriate prescriptions in primary care and correlates with mild cognitive impairment. Pharm Pract (Granada) 2020;18:2017.




34. Duangsong J, Samansaplert P, Khamkong Y, Jenghua K. Use of potentially inappropriate medications for heart failure according to the three sets of heart failure-specific criteria in Thai older patients with heart failure. J Geriatr Cardiol 2022;19:498â510.


35. Zahwe M, Skouri H, Rachidi S, Khoury M, Noureddine S, Ismaâeel H, et al. Potentially inappropriate medications in elderly patients with heart failure: Beers criteria-based study. Int J Pharm Pract 2020;28:652â9.



36. Krittayaphong R, Winijkul A, Methavigul K, Wongtheptien W, Wongvipaporn C, Wisaratapong T, et al. Risk profiles and pattern of antithrombotic use in patients with non-valvular atrial fibrillation in Thailand: a multicenter study. BMC Cardiovasc Disord 2018;18:174.




- TOOLS