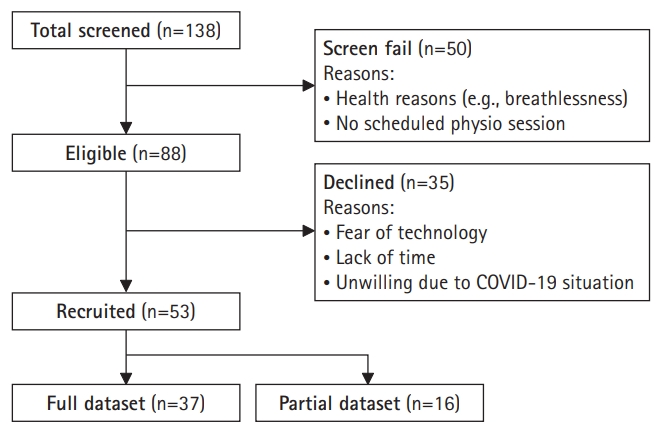
|
 |
- Search
| Ann Geriatr Med Res > Volume 26(2); 2022 > Article |
|
Abstract
Background
Methods
Results
Conclusion
ACKNOWLEDGMENTS
The authors thank Ms. Loo Yen Leng, Ms. Lee Jin Yih, Ms. Audrey Yeo Jing Ping, and Ms. Kalene Pek for their invaluable assistance with this study. We also express our gratitude to the study participants who graciously consented to participate in the study, as well as the doctors, nurses, and physiotherapists of the Falls and Balance Clinic at Tan Tock Seng Hospital who helped us to perform this study.
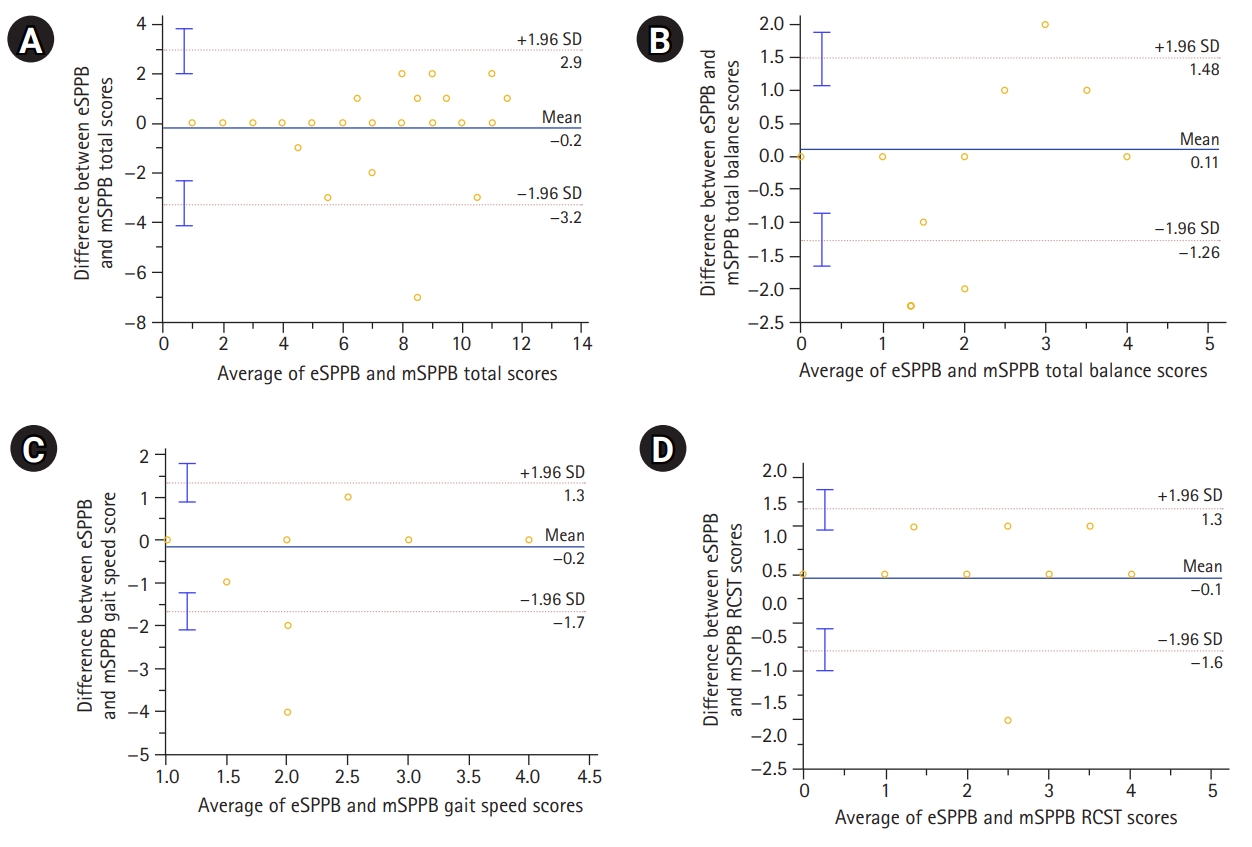
Fig.┬Ā2.
Table┬Ā1.
|
Current study |
Original study (Jung et al.16)) |
|||||
|---|---|---|---|---|---|---|
| Male (n=14) | Female (n=23) | p-value | Male (n=15) | Female (n=25) | p-value | |
| Age (y) | 76.9┬▒6.4 | 79.5┬▒7.0 | 0.451 | 75.9┬▒4.5 | 73.4┬▒6.0 | 0.198 |
| Weight (kg) | 70.1┬▒14.1 | 53.2┬▒11.6 | 0.002* | |||
| Height (m) | 1.6┬▒0.08 | 1.5┬▒0.1 | 0.002* | |||
| BMI (kg/m2) | 26.0┬▒5.0 | 25.0┬▒6.0 | 0.552 | |||
| MBI Total (0ŌĆō100) | 98.0┬▒2.5 | 93.7┬▒7.1 | 0.074 | 96.8┬▒3.2 | 95.7┬▒4.1 | 0.377 |
| iADL (0ŌĆō32) | 19.7┬▒3.2 | 17.6┬▒4.6 | 0.123 | |||
| FRAIL total (0ŌĆō5) | 0.8┬▒0.8 | 1.4┬▒1.1 | 0.304 | 0.5┬▒0.8 | 1.2┬▒1.1 | 0.035** |
| ŌĆāRobust | 6 (43) | 4 (17) | ||||
| ŌĆāPre-frail | 8 (57) | 16 (70) | ||||
| ŌĆāFrail | 0 (0) | 3 (13) | ||||
| FAI total (0ŌĆō45) | 26.4┬▒9.5 | 26.6┬▒11.8 | 0.689 | |||
| BBS total (0ŌĆō56) | 47.2┬▒3.2 | 39.2┬▒11.8 | 0.068 | 53.2┬▒2.5 | 51.4┬▒4.9 | 0.356 |
| FES-I total (16ŌĆō64) | 28.8┬▒11.1 | 29.6┬▒10.2 | 0.919 | |||
| mSPPB (0ŌĆō12) | 7.6┬▒2.8 | 6.0┬▒2.5 | 0.185 | 10.8┬▒1.6 | 9.9┬▒2.4 | 0.161 |
| eSPPB (0ŌĆō12) | 8.1┬▒3.2 | 5.7┬▒3.2 | 0.115 | 10.4┬▒1.7 | 9.9┬▒2.3 | 0.677 |
Values are presented as mean┬▒standard deviation or number (%).
BMI, body mass index; MBI, modified Barthel Index; iADL, instrumental activities of daily living; FRAIL, fatigue, resistance, ambulation, illness, loss of weight; FAI, Frenchay Activities Index; BBS, Berg Balance Scale; FES-I, Falls Efficacy Scale International; mSPPB, manual Short Physical Performance Battery; eSPPB, automated Short Physical Performance Battery.
Table┬Ā2.
| MBI | iADL | FES | FRAIL | FAI | BBS | |
|---|---|---|---|---|---|---|
| mSPPB total | 0.507* | 0.465** | 0.077 | -0.441** | 0.305 | 0.900* |
| ŌĆāBalance | 0.494* | 0.413** | -0.082 | -0.292 | 0.253 | 0.789* |
| ŌĆāGait speed | 0.282 | 0.470** | 0.150 | -0.505* | 0.299 | 0.662* |
| ŌĆāChair stand | 0.430** | 0.269 | 0.121 | -0.297 | 0.201 | 0.718* |
| eSPPB total | 0.508* | 0.530* | 0.138 | -0.383** | 0.362 | 0.869* |
| ŌĆāBalance | 0.569* | 0.499* | 0.100 | -0.239 | 0.380 | 0.831* |
| ŌĆāGait speed | 0.163 | 0.411** | 0.286 | -0.430** | 0.409** | 0.460** |
| ŌĆāChair stand | 0.309 | 0.287 | 0.068 | -0.233 | 0.132 | 0.555* |
Partial correlation adjusted for age and gender.
mSPPB, manual Short Physical Performance Battery; eSPPB, automated Short Physical Performance Battery; MBI, modified Barthel Index; iADL, instrumental activities of daily living; FES, Falls Efficacy Scale; FRAIL, fatigue, resistance, ambulation, illness, loss of weight; FAI, Frenchay Activities Index; BBS, Berg Balance Scale.
Table┬Ā3.
|
mSPPB |
eSPPB |
|||||
|---|---|---|---|---|---|---|
| mSPPB Ōēż8 (n=26) | mSPPB >8 (n =11) | p-value | eSPPB Ōēż8 (n=23) | eSPPB >8 (n=14) | p-value | |
| MBI total | 93.9┬▒6.8 | 98.6┬▒1.8 | 0.091 | 93.3┬▒7.0 | 98.2┬▒2.1 | 0.033** |
| iADL | 17.2┬▒4.4 | 21.1┬▒1.8 | 0.13 | 17.2┬▒4.4 | 20.1┬▒3.2 | 0.115 |
| FRAIL total | 1.5┬▒1.0 | 0.4┬▒0.5 | 0.013** | 1.5┬▒1.0 | 0.6┬▒0.6 | 0.010** |
| FAI total | 24.5┬▒10.8 | 31.2┬▒9.6 | 0.756 | 24.6┬▒11.3 | 27.5┬▒9.1 | 0.972 |
| BBS total | 38.2┬▒10.0 | 50.8┬▒2.9 | 0.008* | 36.6┬▒9.8 | 50.2┬▒2.8 | 0.005* |
| FES-I total | 27.3┬▒9.4 | 34.6┬▒11.6 | 0.343 | 27.4┬▒9.5 | 30.3┬▒9.7 | 0.919 |
| SPPB total (0ŌĆō12) | 5.0┬▒2.4 | 10.5┬▒1.0 | 0.003* | 4.4┬▒2.2 | 10.1┬▒1.3 | 0.001* |
| ŌĆāBalance (0ŌĆō4) | 1.7┬▒1.1 | 3.2┬▒1.0 | 0.003* | 1.5┬▒1.1 | 3.4┬▒0.8 | 0.001* |
| ŌĆāGait speed (0ŌĆō4) | 1.8┬▒0.9 | 3.6┬▒0.7 | 0.007* | 1.6┬▒0.8 | 3.4┬▒0.8 | 0.002* |
| ŌĆāChair stand (0ŌĆō4) | 1.5┬▒1.5 | 3.6┬▒0.5 | 0.003* | 1.3┬▒1.4 | 3.4┬▒1.1 | 0.003* |
Values are presented as mean┬▒standard deviation.
mSPPB, manual Short Physical Performance Battery; eSPPB, automated Short Physical Performance Battery; MBI, modified Barthel Index; iADL, instrumental activities of daily living; FRAIL, fatigue, resistance, ambulation, illness, loss of weight; FAI, Frenchay Activities Index; BBS, Berg Balance Scale; FES-I, Falls Efficacy Scale International.
Table┬Ā4.
|
Current study |
Original study (Jung et al.16)) |
||||||
|---|---|---|---|---|---|---|---|
| mSPPB | eSPPB | p-value | ICC (95% CI) | mSPPB | eSPPB | ICC | |
| Total score (0ŌĆō12) | 6.62┬▒3.26 | 6.57┬▒3.38 | 0.782 | 0.94 (0.88ŌĆō0.97) | 10.2┬▒2.1 | 10.1┬▒2.1 | 0.97 |
| ŌĆāBalance (0ŌĆō4) | 2.14┬▒1.25 | 2.24┬▒1.40 | 0.353 | 0.86 (0.75ŌĆō0.93) | 3.3┬▒1.0 | 3.4┬▒0.7 | 0.77 |
| ŌĆāGait speed (0ŌĆō4) | 2.38┬▒1.16 | 2.32┬▒1.20 | 0.422 | 0.94 (0.89ŌĆō0.97) | 3.4┬▒0.8 | 3.2┬▒0.9 | 0.84 |
| ŌĆāRCST (0ŌĆō4) | 2.11┬▒1.61 | 2.00┬▒1.60 | 0.378 | 0.89 (0.81ŌĆō0.94) | 3.5┬▒1.2 | 3.5┬▒1.2 | 0.99 |
REFERENCES
- TOOLS